- Record: found
- Abstract: found
- Article: found
High Fragmentation Characterizes Tumour-Derived Circulating DNA
Read this article at
Abstract
Background
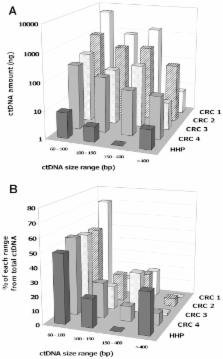
Circulating DNA (ctDNA) is acknowledged as a potential diagnostic tool for various cancers including colorectal cancer, especially when considering the detection of mutations. Certainly due to lack of normalization of the experimental conditions, previous reports present many discrepancies and contradictory data on the analysis of the concentration of total ctDNA and on the proportion of tumour-derived ctDNA fragments.
Methodology
In order to rigorously analyse ctDNA, we thoroughly investigated ctDNA size distribution. We used a highly specific Q-PCR assay and athymic nude mice xenografted with SW620 or HT29 human colon cancer cells, and we correlated our results by examining plasma from metastatic CRC patients.
Conclusion/Significance
Fragmentation and concentration of tumour-derived ctDNA is positively correlated with tumour weight. CtDNA quantification by Q-PCR depends on the amplified target length and is optimal for 60–100 bp fragments. Q-PCR analysis of plasma samples from xenografted mice and cancer patients showed that tumour-derived ctDNA exhibits a specific amount profile based on ctDNA size and significant higher ctDNA fragmentation. Metastatic colorectal patients (n = 12) showed nearly 5-fold higher mean ctDNA fragmentation than healthy individuals (n = 16).
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Circulating nucleic acids (CNAs) and cancer--a survey.
- Record: found
- Abstract: found
- Article: not found