- Record: found
- Abstract: found
- Article: found
The subcortical default mode network and Alzheimer’s disease: a systematic review and meta-analysis

Read this article at
Abstract
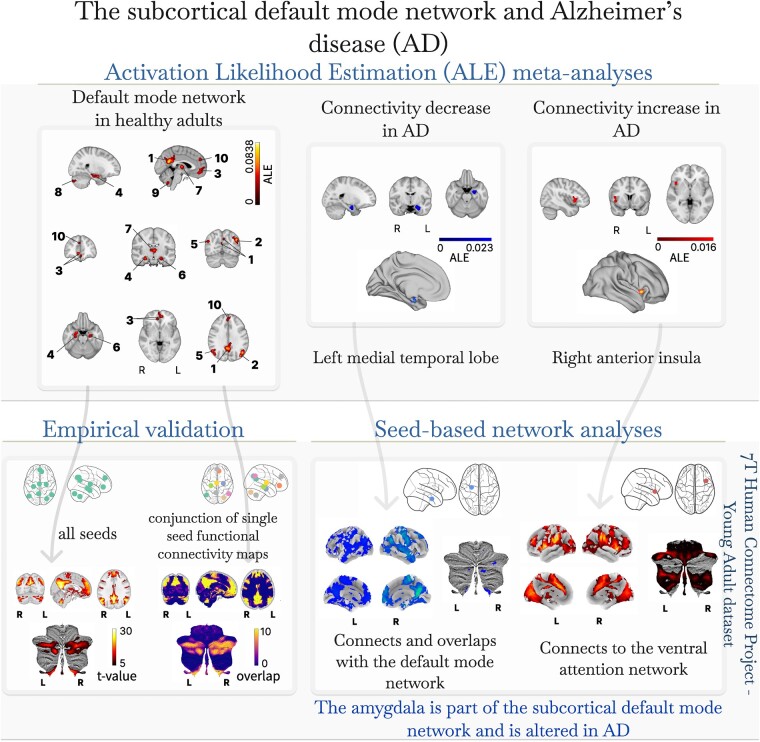
The default mode network is a central cortical brain network suggested to play a major role in several disorders and to be particularly vulnerable to the neuropathological hallmarks of Alzheimer’s disease. Subcortical involvement in the default mode network and its alteration in Alzheimer’s disease remains largely unknown. We performed a systematic review, meta-analysis and empirical validation of the subcortical default mode network in healthy adults, combined with a systematic review, meta-analysis and network analysis of the involvement of subcortical default mode areas in Alzheimer’s disease. Our results show that, besides the well-known cortical default mode network brain regions, the default mode network consistently includes subcortical regions, namely the thalamus, lobule and vermis IX and right Crus I/II of the cerebellum and the amygdala. Network analysis also suggests the involvement of the caudate nucleus. In Alzheimer’s disease, we observed a left-lateralized cluster of decrease in functional connectivity which covered the medial temporal lobe and amygdala and showed overlap with the default mode network in a portion covering parts of the left anterior hippocampus and left amygdala. We also found an increase in functional connectivity in the right anterior insula. These results confirm the consistency of subcortical contributions to the default mode network in healthy adults and highlight the relevance of the subcortical default mode network alteration in Alzheimer’s disease.
Abstract
Seoane et al. find the most consistently identified subcortical default mode network in healthy adults and its disruption in Alzheimer’s disease. Through systematic review, meta-analysis and network analysis, they identify a left-lateralized functional connectivity decrease, particularly in the medial temporal lobe and amygdala, in Alzheimer’s disease.
Graphical Abstract
Related collections
Most cited references144
- Record: found
- Abstract: found
- Article: not found
The organization of the human cerebral cortex estimated by intrinsic functional connectivity.
- Record: found
- Abstract: found
- Article: not found