- Record: found
- Abstract: found
- Article: found
Nanosuspension-Based Drug Delivery Systems for Topical Applications
Read this article at
Abstract
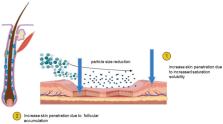
Nanosuspensions have garnered recent attention as a promising strategy for mitigating the bioavailability challenges of hydrophobic drugs, particularly those characterized by poor solubility in both aqueous and organic environments. Addressing solubility issues associated with poorly water-soluble drugs has largely resolved the need to enhance drug absorption and bioavailability. As mucosal formulations and topical administration progress in the future, nanosuspension drug delivery, straightforward formulation techniques, and versatile applications will continue to be subjects of interest. Nanosuspensions have undergone extensive scrutiny in preparation for topical applications, encompassing ocular, pulmonary, and dermal usage. Among the numerous methods aimed at improving cutaneous application, nanocrystals represent a relatively recent yet profoundly intriguing approach. Despite the increasing availability of various nanosuspension products, primarily designed for oral administration, only a limited number of studies have explored skin permeability and drug accumulation in the context of nanosuspensions. Nevertheless, the scant published research unequivocally underscores the potential of this approach for enhancing cutaneous bioavailability, particularly for active ingredients with low to medium solubility. Nanocrystals exhibit increased skin adhesiveness in addition to heightened saturation solubility and dissolution rate, thereby augmenting cutaneous distribution. The article provides a comprehensive overview of nanosuspensions for topical application. The methodology employed is robust, with a well-defined experimental design; however, the limited sample size raises concerns about the generalizability of the findings. While the results demonstrate promising outcomes in terms of enhanced drug delivery, the discussion falls short of addressing certain limitations. Additionally, the references largely focus on recent studies, but a more diverse inclusion of historical perspectives could offer a more holistic view of the subject.
Related collections
Most cited references113
- Record: found
- Abstract: found
- Article: found
Liposomes: structure, composition, types, and clinical applications ☆

- Record: found
- Abstract: found
- Article: found
Emerging role of nanosuspensions in drug delivery systems
- Record: found
- Abstract: found
- Article: not found