- Record: found
- Abstract: found
- Article: found
Prophylactic Aminophylline for Prevention of Apnea at Higher-Risk Preterm Neonates
Read this article at
Abstract
Objectives:
This study aimed to assess the safety and prophylactic effects of aminophylline on the incidence of apnea in premature neonates.
Patients and Methods:
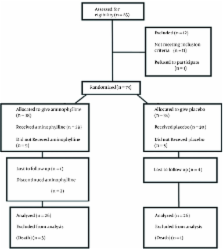
This study was a randomized clinical trial (RCT) research. The prophylactic effect of aminophylline on apnea was investigated in premature babies in our NICU (IRAN-Isfahan). In the study group (A), 5 mg/kg aminophylline was initially administered as a loading dose. Then, every 8 hours, 1.5 mg/kg was given as maintenance dose for the next 10 days. In the control group (C), no aminophylline was used during the first ten days of life.
Results:
Fifty-two neonates were randomized for the study and all of them completed it. Primary outcomes were clearly different between the two groups. Only 2 infants (7.7%) who had been placed in aminophylline group developed apnea, as compared to 16 infants (61.5%) in the control group (P < 0.001). Three and four neonates (11.5%, 15.4%) in the aminophylline group developed bradycardia and cyanosis respectively, as compared to 16 infants (61.5%) who did not receive aminophylline (P < 0.001). Median time of need to NCPAP (Nasal Continuous Positive Airway Pressure) was 1 (0 - 4) days and 2.5 (0.5 - 6.5) days in group A and C, respectively (P = 0.03). No side effects were reported in neonates (P > 0.999). Median time of hospitalization was shorter in aminophylline group (P = 0.04).
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
Caffeine therapy for apnea of prematurity.
- Record: found
- Abstract: found
- Article: not found
Apnea, sudden infant death syndrome, and home monitoring.
- Record: found
- Abstract: found
- Article: not found