- Record: found
- Abstract: found
- Article: not found
Engineering strategies to overcome the current roadblocks in CAR T cell therapy
Read this article at
Abstract
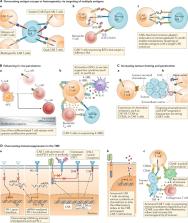
T cells genetically engineered to express chimeric antigen receptors (CARs) have proven — and impressive — therapeutic activity in patients with certain subtypes of B cell leukaemia or lymphoma, with promising efficacy also demonstrated in patients with multiple myeloma. Nevertheless, various barriers restrict the efficacy and/or prevent the widespread use of CAR T cell therapies in these patients as well as in those with other cancers, particularly solid tumours. Key challenges relating to CAR T cells include severe toxicities, restricted trafficking to, infiltration into and activation within tumours, suboptimal persistence in vivo, antigen escape and heterogeneity, and manufacturing issues. The evolution of CAR designs beyond the conventional structures will be necessary to address these limitations and to expand the use of CAR T cells to a wider range of malignancies. Investigators are addressing the current obstacles with a wide range of engineering strategies in order to improve the safety, efficacy and applicability of this therapeutic modality. In this Review, we discuss the innovative designs of novel CAR T cell products that are being developed to increase and expand the clinical benefits of these treatments in patients with diverse cancers.
Abstract
Chimeric antigen receptor (CAR) T cell therapy, the first approved therapeutic approach with a genetic engineering component, holds substantial promise in the treatment of a range of cancers but is nevertheless limited by various challenges, including toxicities, intrinsic and acquired resistance mechanisms, and manufacturing issues. In this Review, the authors describe the innovative approaches to the engineering of CAR T cell products that are providing solutions to these challenges and therefore have the potential to considerably improve the safety and effectiveness of treatment.
Key points
-
Chimeric antigen receptor (CAR) T cells have induced remarkable responses in patients with certain haematological malignancies, yet various barriers restrict the efficacy and/or prevent the widespread use of this treatment.
-
Investigators are addressing these challenges with engineering strategies designed to improve the safety, efficacy and applicability of CAR T cell therapy.
-
CARs have modular components, and therefore the optimal molecular design of the CAR can be achieved through many variations of the constituent protein domains.
-
Toxicities currently associated with CAR T cell therapy can be mitigated using engineering strategies to make CAR T cells safer and that potentially broaden the range of tumour-associated antigens that can be targeted by overcoming on-target, off-tumour toxicities.
-
CAR T cell efficacy can be enhanced by using engineering strategies to address the various challenges relating to the unique biology of diverse haematological and solid malignancies.
-
Strategies to address the manufacturing challenges can lead to an improved CAR T cell product for all patients.
Related collections
Most cited references166
- Record: found
- Abstract: found
- Article: not found
Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy.
- Record: found
- Abstract: found
- Article: not found
Inducible apoptosis as a safety switch for adoptive cell therapy.
- Record: found
- Abstract: found
- Article: not found
