- Record: found
- Abstract: found
- Article: found
CAR-T cell therapy: current limitations and potential strategies
Read this article at
Abstract
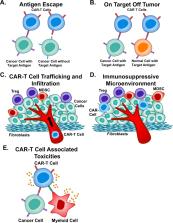
Chimeric antigen receptor (CAR)-T cell therapy is a revolutionary new pillar in cancer treatment. Although treatment with CAR-T cells has produced remarkable clinical responses with certain subsets of B cell leukemia or lymphoma, many challenges limit the therapeutic efficacy of CAR-T cells in solid tumors and hematological malignancies. Barriers to effective CAR-T cell therapy include severe life-threatening toxicities, modest anti-tumor activity, antigen escape, restricted trafficking, and limited tumor infiltration. In addition, the host and tumor microenvironment interactions with CAR-T cells critically alter CAR-T cell function. Furthermore, a complex workforce is required to develop and implement these treatments. In order to overcome these significant challenges, innovative strategies and approaches to engineer more powerful CAR-T cells with improved anti-tumor activity and decreased toxicity are necessary. In this review, we discuss recent innovations in CAR-T cell engineering to improve clinical efficacy in both hematological malignancy and solid tumors and strategies to overcome limitations of CAR-T cell therapy in both hematological malignancy and solid tumors.
Related collections
Most cited references106
- Record: found
- Abstract: found
- Article: not found
Microenvironmental regulation of tumor progression and metastasis.
- Record: found
- Abstract: found
- Article: not found
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
- Record: found
- Abstract: found
- Article: not found