- Record: found
- Abstract: found
- Article: found
Age-associated changes in amyloid-β and formaldehyde concentrations in cerebrospinal fluid of rhesus monkeys
letter
July 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
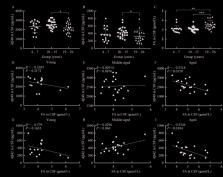
A: Intergroup analyses of Aβ40 concentrations. B: Intergroup analyses of Aβ42 concentrations.
C: Intergroup analyses of FA concentrations. D: Correlation between CSF Aβ40 and FA
concentrations in young group (R=–0.3385, P=0.2172). E: Correlation between CSF Aβ40
and FA concentrations in middle-aged group (R=0.02914, P=0.8976). F: Correlation between
CSF Aβ40 and FA concentrations in aged group (R=–0.5318, P=0.0158). G: Correlation
between CSF Aβ42 and FA concentrations in young group (R=–0.379, P=0.1635). H: Correlation
between CSF Aβ42 and FA concentrations in middle-aged group (R=0.4296, P=0.046). I:
Correlation between CSF Aβ42 and FA concentrations in aged group (R=–0.5344, P=0.0184).
Error bars indicate mean± standard deviation (SD). *: P<0.05, **:P<0.01, ***:P<0.001.
Aβ: β-amyloid; CSF: Cerebrospinal fluid.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Methanol and formic acid toxicity: biochemical mechanisms.
Jyrki Liesivuori, and Savolainen (1991)
- Record: found
- Abstract: found
- Article: not found
Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease.
Nu-Fang Fang, Lan Guo, Doris Chen … (2011)
- Record: found
- Abstract: found
- Article: not found
Accumulated hippocampal formaldehyde induces age-dependent memory decline.
J. Zhou, Hongjun Luo, Xiaohui Wang … (2013)