- Record: found
- Abstract: found
- Article: found
Formaldehyde and De/Methylation in Age-Related Cognitive Impairment
Read this article at
Abstract
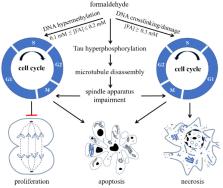
Formaldehyde (FA) is a highly reactive substance that is ubiquitous in the environment and is usually considered as a pollutant. In the human body, FA is a product of various metabolic pathways and participates in one-carbon cycle, which provides carbon for the synthesis and modification of bio-compounds, such as DNA, RNA, and amino acids. Endogenous FA plays a role in epigenetic regulation, especially in the methylation and demethylation of DNA, histones, and RNA. Recently, epigenetic alterations associated with FA dysmetabolism have been considered as one of the important features in age-related cognitive impairment (ARCI), suggesting the potential of using FA as a diagnostic biomarker of ARCI. Notably, FA plays multifaceted roles, and, at certain concentrations, it promotes cell proliferation, enhances memory formation, and elongates life span, effects that could also be involved in the aetiology of ARCI. Further investigation of and the regulation of the epigenetics landscape may provide new insights about the aetiology of ARCI and provide novel therapeutic targets.
Related collections
Most cited references120
- Record: found
- Abstract: found
- Article: not found
DNA methylation and its basic function.
- Record: found
- Abstract: found
- Article: not found
Alzheimer's disease
- Record: found
- Abstract: found
- Article: not found