- Record: found
- Abstract: found
- Article: found
Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming

Read this article at
Abstract
During the process of reprogramming to induced pluripotent stem (iPS) cells, somatic cells switch from oxidative to glycolytic metabolism, a transition associated with profound mitochondrial reorganization. Neither the importance of mitochondrial remodelling for cell reprogramming, nor the molecular mechanisms controlling this process are well understood. Here, we show that an early wave of mitochondrial fragmentation occurs upon expression of reprogramming factors. Reprogramming-induced mitochondrial fission is associated with a minor decrease in mitochondrial mass but not with mitophagy. The pro-fission factor Drp1 is phosphorylated early in reprogramming, and its knockdown and inhibition impairs both mitochondrial fragmentation and generation of iPS cell colonies. Drp1 phosphorylation depends on Erk activation in early reprogramming, which occurs, at least in part, due to downregulation of the MAP kinase phosphatase Dusp6. Taken together, our data indicate that mitochondrial fission controlled by an Erk-Drp1 axis constitutes an early and necessary step in the reprogramming process to pluripotency.
Abstract
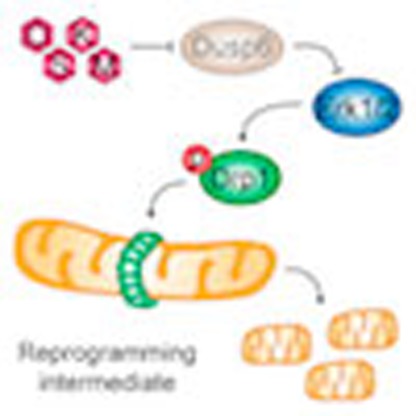 Reprogramming of somatic cells is a stepwise process where cells must overcome several
barriers before reaching the pluripotent state. Here the authors show that mitochondrial
fission in response to ERK1/2 signalling is an important early step during reprogramming
to pluripotency.
Reprogramming of somatic cells is a stepwise process where cells must overcome several
barriers before reaching the pluripotent state. Here the authors show that mitochondrial
fission in response to ERK1/2 signalling is an important early step during reprogramming
to pluripotency.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis.
- Record: found
- Abstract: found
- Article: not found
FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment.
- Record: found
- Abstract: found
- Article: not found