- Record: found
- Abstract: found
- Article: found
Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis
Read this article at
Abstract
Objective
To evaluate the efficacy and safety of artificial pancreas treatment in non-pregnant outpatients with type 1 diabetes.
Eligibility criteria for selecting studies
Randomised controlled trials in non-pregnant outpatients with type 1 diabetes that compared the use of any artificial pancreas system with any type of insulin based treatment. Primary outcome was proportion (%) of time that sensor glucose level was within the near normoglycaemic range (3.9-10 mmol/L). Secondary outcomes included proportion (%) of time that sensor glucose level was above 10 mmol/L or below 3.9 mmol/L, low blood glucose index overnight, mean sensor glucose level, total daily insulin needs, and glycated haemoglobin. The Cochrane Collaboration risk of bias tool was used to assess study quality.
Results
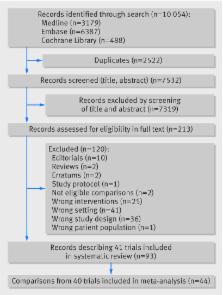
40 studies (1027 participants with data for 44 comparisons) were included in the meta-analysis. 35 comparisons assessed a single hormone artificial pancreas system, whereas nine comparisons assessed a dual hormone system. Only nine studies were at low risk of bias. Proportion of time in the near normoglycaemic range (3.9-10.0 mmol/L) was significantly higher with artificial pancreas use, both overnight (weighted mean difference 15.15%, 95% confidence interval 12.21% to 18.09%) and over a 24 hour period (9.62%, 7.54% to 11.7%). Artificial pancreas systems had a favourable effect on the proportion of time with sensor glucose level above 10 mmol/L (−8.52%, −11.14% to −5.9%) or below 3.9 mmol/L (−1.49%, −1.86% to −1.11%) over 24 hours, compared with control treatment. Robustness of findings for the primary outcome was verified in sensitivity analyses, by including only trials at low risk of bias (11.64%, 9.1% to 14.18%) or trials under unsupervised, normal living conditions (10.42%, 8.63% to 12.2%). Results were consistent in a subgroup analysis both for single hormone and dual hormone artificial pancreas systems.
Conclusions
Artificial pancreas systems are an efficacious and safe approach for treating outpatients with type 1 diabetes. The main limitations of current research evidence on artificial pancreas systems are related to inconsistency in outcome reporting, small sample size, and short follow-up duration of individual trials.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity.
- Record: found
- Abstract: found
- Article: not found
Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes.
- Record: found
- Abstract: found
- Article: not found