- Record: found
- Abstract: found
- Article: found
Decavanadate Salts of Cytosine and Metformin: A Combined Experimental-Theoretical Study of Potential Metallodrugs Against Diabetes and Cancer
Read this article at
Abstract
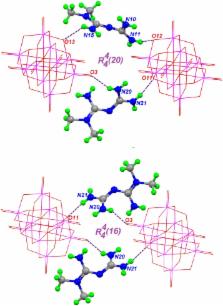
Cytosine, a DNA and RNA building-block, and Metformin, the most widely prescribed drug for the treatment of Type 2 Diabetes mellitus were made to react separately with ammonium or sodium metavanadates in acidic aqueous solutions to obtain two polyoxovanadate salts with a 6:1 ratio of cation-anion. Thus, compounds [HCyt] 6[V 10O 28]·4H 2O, 1 and [HMetf] 6[V 10O 28]·6H 2O, 2 (where HCyt = Cytosinium cation, [C 4H 6N 3O] + and HMetf = Metforminium cation, [C 4H 12N 5] +) were obtained and characterized by elemental analysis, single crystal X-ray diffraction, vibrational spectroscopy (IR and Raman), solution 51V-NMR, thermogravimetric analysis (TGA-DTGA), as well as, theoretical methods. Both compounds crystallized in P space group with Z' = 1/2, where the anionic charge of the centrosymmetric ion [V 10O 28] 6− is balanced by six Cytosinium and six Metforminium counterions, respectively. Compound 1 is stabilized by π-π stacking interactions coming from the aromatic rings of HCyt cations, as denoted by close contacts of 3.63 Å. On the other hand, guanidinium moieties from the non-planar HMetf in Compound 2 interact with decavanadate μ 2-O atoms via N−H···O hydrogen bonds. The vibrational spectroscopic data of both IR and Raman spectra show that the dominant bands in the 1000-450 cm −1 range are due to the symmetric and asymmetric ν (V−O) vibrational modes. In solution, 51V-NMR experiments of both compounds show that polyoxovanadate species are progressively transformed into the monomeric, dimeric and tetrameric oxovanadates. The thermal stability behavior suggests a similar molecular mechanism regarding the loss of water molecules and the decomposition of the organic counterions. Yet, no changes were observed in the TGA range of 540–580°C due to the stability of the [V 10O 28] 6− fragment. Dispersion-corrected density functional theory (DFT-D) calculations were carried out to model the compounds in aqueous phase using a polarized continuum model calculation. Optimized structures were obtained and the main non-covalent interactions were characterized. Biological activities of these compounds are also under investigation. The combination of two therapeutic agents opens up a window toward the generation of potential metalopharmaceuticals with new and exciting pharmacological properties.
Related collections
Most cited references85
- Record: found
- Abstract: not found
- Article: not found
Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg
- Record: found
- Abstract: found
- Article: not found
Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I.
- Record: found
- Abstract: not found
- Article: not found