- Record: found
- Abstract: found
- Article: not found
Asymmetric synthesis from terminal alkenes by diboration/cross-coupling cascades
Read this article at
Abstract
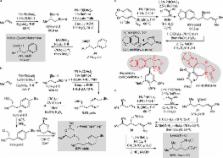
Amongst prospective starting materials for organic synthesis, terminal (monosubstituted) alkenes are ideal. In the form of α-olefins, they are manufactured on enormous scale and they are the core product features from many organic chemical reactions. While their latent reactivity can easily enable hydrocarbon chain extension, alkenes also have the attractive feature of being stable in the presence of many acids, bases, oxidants and reductants. In spite of these impressive attributes, relatively few catalytic enantioselective transformations have been developed that transform aliphatic α-olefins in >90% ee and, with the exception of site-controlled isotactic polymerization of α-olefins, 1 none of these processes result in chain-extending C-C bond formation to the terminal carbon. 2, 3, 4, 5, 6 Herein, we describe a strategy that directly addresses this gap in synthetic methodology and present a single-flask catalytic enantioselective conversion of terminal alkenes into a range of chiral products. These reactions are enabled by an unusual neighboring group participation effect that accelerates Pd-catalyzed cross-coupling of 1,2-bis(boronates) relative to nonfunctionalized alkyl boronate analogs. In tandem with enantioselective diboration, this reactivity feature connects abundant alkene starting materials to a diverse array of chiral products. Importantly with respect to synthesis utility, the tandem diboration/cross-coupling reaction (DCC reaction) generally provides products in high yield and high selectivity (>95:5 enantiomer ratio), employs low loadings (1–2 mol %) of commercially available catalysts and reagents, it offers an expansive substrate scope, and can address a broad range of alcohol and amine synthesis targets, many of which cannot be easily addressed with current technology.
Related collections
Most cited references28
- Record: found
- Abstract: not found
- Article: not found
Selectivity in propene polymerization with metallocene catalysts.
- Record: found
- Abstract: not found
- Article: not found
Catalytic enantioselective allylation of carbonyl compounds and imines.
- Record: found
- Abstract: found
- Article: not found
