- Record: found
- Abstract: found
- Article: found
A universal stress protein upregulated by hypoxia has a role in Burkholderia cenocepacia intramacrophage survival: Implications for chronic infection in cystic fibrosis
Read this article at
Abstract
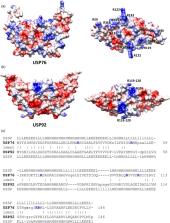
Universal stress proteins (USPs) are ubiquitously expressed in bacteria, archaea, and eukaryotes and play a lead role in adaptation to environmental conditions. They enable adaptation of bacterial pathogens to the conditions encountered in the human niche, including hypoxia, oxidative stress, osmotic stress, nutrient deficiency, or acid stress, thereby facilitating colonization. We previously reported that all six USP proteins encoded within a low‐oxygen activated ( lxa) locus in Burkholderia cenocepacia showed increased abundance during chronic colonization of the cystic fibrosis (CF) lung. However, the role of USPs in chronic cystic fibrosis infection is not well understood. Structural modeling identified surface arginines on one lxa‐encoded USP, USP76, which suggested it mediated interactions with heparan sulfate. Using mutants derived from the B. cenocepacia strain, K56‐2, we show that USP76 is involved in host cell attachment. Pretreatment of lung epithelial cells with heparanase reduced the binding of the wild‐type and complement strains but not the Δ usp76 mutant strain, indicating that USP76 is directly or indirectly involved in receptor recognition on the surface of epithelial cells. We also show that USP76 is required for growth and survival in many conditions associated with the CF lung, including acidic conditions and oxidative stress. Moreover, USP76 also has a role in survival in macrophages isolated from people with CF. Overall, while further elucidation of the exact mechanism(s) is required, we can conclude that USP76, which is upregulated during chronic infection, is involved in bacterial survival within CF macrophages, a hallmark of Burkholderia infection.
Abstract
A Burkholderia cenocepacia universal stress protein encoded on the BCAM0276 gene (USP76) which is upregulated in chronic infection in people with cystic fibrosis (CF) and by hypoxia, is involved in attachment to human epithelial cells. It is required for growth in conditions associated with the CF lung, including acidic conditions and oxidative stress. Survival of usp76 deletion mutant was impaired in monocyte‐derived macrophages from CF patients, suggesting it is involved in bacterial survival within macrophages, a hallmark of Burkholderia infection.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.
- Record: found
- Abstract: found
- Article: not found
Twilight zone of protein sequence alignments.
- Record: found
- Abstract: found
- Article: not found
