- Record: found
- Abstract: found
- Article: found
Tumor Cell-Based Vaccine Generated With High Hydrostatic Pressure Synergizes With Radiotherapy by Generating a Favorable Anti-tumor Immune Microenvironment
Read this article at
Abstract
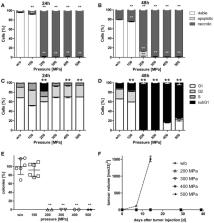
Dendritic cell (DC)-based vaccines pulsed with high hydrostatic pressure (HHP)-inactivated tumor cells have been demonstrated to be a promising immunotherapy for solid tumors. We focused on sole injection of tumor cells that were inactivated by HHP and their combination with local radiotherapy (RTx) for in vivo induction of anti-tumor immune responses. HHP-treatment of tumor cells resulted in pre-dominantly necrotic cells with degraded DNA. We confirmed that treatments at 200 MPa or higher completely inhibited the formation of tumor cell colonies in vitro. No tumor growth was seen in vivo after injection of HHP-treated tumor cells. Single vaccination with HHP-killed tumor cells combined with local RTx significantly retarded tumor growth and improved the survival as shown in B16-F10 and CT26 tumor models. In B16-F10 tumors that were irradiated with 2 × 5Gy and vaccinated once with HHP-killed tumor cells, the amount of natural killer (NK) cells, monocytes/macrophages, CD4+ T cells and NKT cells was significantly increased, while the amount of B cells was significantly decreased. In both models, a trend of increased CD8+ T cell infiltration was observed. Generally, in irradiated tumors high amounts of CD4+ and CD8+ T cells expressing PD-1 were found. We conclude that HHP generates inactivated tumor cells that can be used as a tumor vaccine. Moreover, we show for the first time that tumor cell-based vaccine acts synergistically with RTx to significantly retard tumor growth by generating a favorable anti-tumor immune microenvironment.
Related collections
Most cited references39
- Record: found
- Abstract: not found
- Article: not found
A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V
- Record: found
- Abstract: found
- Article: not found
Clinical use of dendritic cells for cancer therapy.
- Record: found
- Abstract: found
- Article: not found