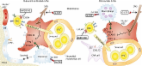
Somatic stem cells are present in most tissues of the adult body. They control the continuous production of differentiated cell types in highly regenerative tissues such as the gastrointestinal mucosa, skin epidermis, and blood. They also play a critical role in response to tissue injury, during which they become actively engaged in repair processes. The self-renewal and differentiation activity of stem cells is controlled by their surrounding microenvironment, which is known as the stem cell niche. Although the concept of niches is well accepted and experimentally proven in various invertebrate systems, mammalian stem cell niches remain poorly understood, as the precise location of the stem cells themselves often remains elusive. One of the most well-characterized somatic stem cells in mammals is the mouse hematopoietic stem cell (HSC), which resides in the bone marrow. Using multiparameter flow cytometry, linnegSca1hic-Kit+CD34−CD48−CD150hi HSCs can be identified and isolated prospectively. At the clonal level, these cells can reconstitute the entire hematopoietic system of lethally irradiated mice and are serially transplantable (Purton and Scadden, 2007; Wilson et al., 2007). Because HSCs are preferentially found as single cells in the trabecular cavities of long bones, their niche location is assumed to be nearby. However, there is significant debate about the more detailed location of HSCs within this area, which contains not only the endosteal region, located in the immediate proximity of the bone lining osteoblasts (OBs), but also a highly vascularized area toward the center of the bone marrow (Fig. 1). Several studies have attempted to localize HSCs in bone sections or by using confocal/two-photon intravital imaging based on three-color fluorescence microscopy (Lord and Hendry, 1972; Nilsson et al., 2001; Lo Celso et al., 2009; Xie et al., 2009). However, these techniques do not define resident HSCs as accurately as eight-parameter flow cytometry, admitting the possibility that early progenitors rather than bona fide HSCs were imaged. Nevertheless, putative HSCs have been found near the endosteum lined by OBs (endosteal niche) or in association with sinusoidal endothelium (perivascular niche; Fig. 1 and Fig. 2). Importantly, sinusoids are also found close to the endosteum, but are more abundant at greater distances from the bone surfaces (Wilson and Trumpp, 2006; Kiel and Morrison, 2008). Thus, the identity and composition of the niche housing functional HSCs remains unclear. Figure 1. Location of HSC niches in trabecular bone cavities. HSCs are located at the endosteum, which is lined by OBs and is remodeled by osteoclasts. OBs promote HSC maintenance. Vascular sinusoids are found close to the endosteum, but more frequently at greater distances. HSCs are also situated nearby sinusoids toward the center of the marrow. Perivascular nestin+ MSCs and the more abundant CAR cells promote HSC maintenance. It is also possible that there is more than one niche, and that HSCs are not static, but instead dynamically change their niche location in response to injury or to feedback signals (Trumpp et al., 2010). Directly relating to this point, it must be noted that several of the studies designed to identify the location of the HSC niche make use of mice that have received total body irradiation. Under these conditions, it is highly likely that the niche is undergoing a dynamic remodeling process and may not be identical to the homeostatic HSC niche. Moreover, the location of some important cellular components of the niches may not be restricted to the endosteum or the perivascular niche area, but may be part of both environments, raising the possibility that both niches and the location of HSCs may not be as distinct as is currently assumed. Mesenchymal stem cells (MSCs): the main niche players Support for the hypothesis that one cell type is present at various sites within the marrow niche was obtained by a recent landmark study by Méndez-Ferrer et al. (2010). This group identified a stromal nestin-expressing MSC population (nestin+ MSC) that is closely associated with putative HSCs. Nestin+ MSCs are strictly perivascular and are typically found in more central areas of the marrow, but they are also present in the immediate vicinity of the endosteum, albeit at lower frequency. Moreover, nestin+ MSCs are tightly associated with adrenergic nerve fibers of the sympathetic nervous system (SNS) that regulate HSC mobilization and are responsible for the circadian oscillations in circulating HSC numbers (Katayama et al., 2006; Méndez-Ferrer et al., 2008). Strikingly, these MSCs express higher levels of HSC maintenance factor transcripts, including CXCL12, stem cell factor (SCF), angiopoietin-1 (Ang-1), IL-7, vascular cell adhesion molecule 1 (VCAM1), and osteopontin (OPN), compared with any other stromal cell type including OBs. Depletion of nestin+ MSCs by inducible expression of diphtheria toxin receptor (DTR) in nestin-expressing cells caused the mobilization of ∼50% of HSCs to the spleen. In addition, the homing of transplanted progenitor cells into MSC-depleted recipients was reduced by 90%. Moreover, treatment with the HSC-mobilizing factor granulocyte colony-stimulating factor (G-CSF) decreased the expression of CXCL12 and other factors required for HSC maintenance and retention by nestin+ MSCs. These cells also seem to mediate the capacity of parathyroid hormone to increase HSC numbers, which had previously been correlated with an increase in putative niche OBs (Calvi et al., 2003; Adams et al., 2007). Parathyroid hormone directly stimulated proliferation of nestin+ MSCs while simultaneously promoting their differentiation into OBs (Méndez-Ferrer et al., 2010). Collectively, these data support the hypothesis that nestin+ MSCs are a functional component of the bone marrow HSC niche. Most interestingly, nestin+ MSCs show several similarities to recently identified mesenchymal progenitors (Sugiyama et al., 2006; Omatsu et al., 2010). These bipotent adipoosteogenic progenitors were identified in a mouse strain in which GFP was expressed from the endogenous CXCL12 locus (Sugiyama et al., 2006; Omatsu et al., 2010). Because of their high CXCL12 expression and their long cellular processes, these cells were named CXCL12-abundant reticular (CAR) cells. The majority of putative HSCs are found in close proximity to CAR cells by immunohistochemistry, and like nestin+ MSCs, CAR cells are predominantly found in the more central areas of the marrow, with some also located close to vessels near the endosteum (Fig. 1). Although CAR cells are more abundant than nestin+ MSCs, they too are tightly associated with sinusoidal endothelium and have a similar morphology to vascular pericytes. These data are in agreement with studies in humans, suggesting that virtually all MSC activity is found within the larger pericyte population that associates closely with the vascular system in the entire body (Crisan et al., 2008). Similar to nestin+ MSCs, CAR cells express HSC maintenance proteins such as CXCL12 and SCF. Induced depletion of CAR cells using a DTR approach causes a partial loss of HSC activity associated with a decrease in HSC cycling, suggesting that CAR cells promote HSC cycling and self-renewal. Even though this phenomenon was not observed after depletion of nestin+ MSCs, it seems likely that CAR cells and nestin+ MSCs represent two highly overlapping CXCL12-expressing cell populations. Because nestin+ MSCs are approximately four times less abundant than CAR cells, contain all colony-forming-unit fibroblast activity within the marrow, harbor high self-renewal activity in vitro and in vivo, and are capable of multilineage differentiation into bone, cartilage, and fat, it appears that nestin+ MSCs may represent a more primitive population compared with CAR cells and may even be a CAR subpopulation. As it remains unclear whether nestin+ MSCs are homogeneous and whether they all express high levels of CXCL12, it is possible that some nestin+ MSCs may not be CAR cells. Although future studies will need to dissect the cellular relationship between these two niche cell populations, there is now convincing evidence that perivascular mesenchymal stem/progenitor cells are critical inhabitants of the HSC niche. OBs and the endosteal niche Nestin+ MSCs and CAR cells are able to generate osteoprogenitor intermediates and, eventually, OBs, which line the bone surface at the endosteum. This was the first location to be proposed as the putative HSC niche, and HSCs at the endosteum have subsequently been identified by confocal/two-photon intravital imaging (Lord and Hendry, 1972; Nilsson et al., 2001; Calvi et al., 2003; Zhang et al., 2003). HSCs isolated from endosteal regions by FACS show higher reconstitution activity compared with phenotypically identical cells from the center of the bone marrow (Grassinger et al., 2010). The cell type initially suggested to be critical within the so-called endosteal niche was the bone-lining, spindle-shaped OB, which displays a CD45−CD31−TER119−Sca1−CD51+ phenotype. Induced depletion of OBs causes mobilization of HSCs/progenitors to the spleen, and some genetically modified mice with augmented OBs show a simultaneous increase in HSC numbers (Calvi et al., 2003; Zhang et al., 2003; Visnjic et al., 2004; Zhu et al., 2007). Furthermore, a population of CD45−CD105+ osteoprogenitor cells from fetal long bone or calvaria is sufficient to generate an HSC niche when transplanted under the kidney capsule (Chan et al., 2009). Similarly, adult human bone marrow contains a population of self-renewing CD45−CD146+ perivascular osteoprogenitors that can generate bone and marrow when transplanted under the skin of immunodeficient mice (Sacchetti et al., 2007). Moreover, HSCs express the calcium ion receptor, which has been demonstrated to be important for HSC engraftment at the endosteum by enabling HSCs to follow the Ca2+ gradient that results from bone remodeling processes occurring at the endosteum (Adams et al., 2006). Finally, OBs produce factors that are known to be involved in HSC retention and maintenance, including CXCL12, OPN, and N-cadherin, in addition to factors that keep HSCs in a quiescent state, including Ang-1, membrane-bound SCF, and thrombopoietin (TPO; Arai et al., 2004; Yoshihara et al., 2007; Thoren et al., 2008). The identification of perivascular MSC/CAR cells as key niche cells now calls into question the role of OBs as HSC niche components, as MSCs generate OBs that remain in the close vicinity but may not contribute directly to the niche activity. Accordingly, a recent study suggests that osterix+ osteoprogenitors rather than mature osteocalcin+ OBs are required for the integrity of the niche (Raaijmakers et al., 2010). In addition, MSCs seem to express much higher levels of some HSC maintenance factors compared with OBs (CXCL12, SCF, IL-7, VCAM1, and OPN; Méndez-Ferrer et al., 2010). However, to what degree the expression levels of these factors influence niche activity remains unclear. Importantly, sinusoidal endothelium with associated MSC/CAR cells is also found directly at the endosteum. This raises the possibility that endosteal HSCs, although being close to OBs, are still part of a perivascular niche with OBs playing no direct role in HSC function. Conversely, some bone marrow HSCs localized far away from bone surfaces might simply have been caught in histological sections during migration from a niche to a vessel (Wright et al., 2001). Moreover, mobilized HSCs present at extramedullary sites like the spleen have no contact with osteoblastic cells, although whether the splenic environment maintains HSC self-renewal as well as the bone marrow remains questionable. Although it has been suggested that the endosteum is not important for HSC function, it may be too early to dismiss OBs as critical niche components (Kiel et al., 2005; Méndez-Ferrer et al., 2010). OBs express several factors important for HSC function, including membrane-bound SCF, and they seem to be the exclusive source of TPO (Arai et al., 2009). Importantly, TPO signaling via the c-MPL receptor has been shown to mediate HSC quiescence (Qian et al., 2007; Yoshihara et al., 2007), which is a typical feature of the most potent HSCs in steady-state bone marrow (Wilson et al., 2008). Moreover, although c-MPL−/− mice are born with normal numbers of HSCs, their frequency progressively declines with age, demonstrating a critical role for OB-derived TPO in adult HSC maintenance in vivo (Qian et al., 2007; Yoshihara et al., 2007). How can the two aforementioned niche concepts be combined to form a comprehensive picture of HSC niches? Although various putative niche cell types have been experimentally ablated using the DTR or thymidine kinase systems, in none of these experiments (MSC, CAR, or OB) did HSCs disappear completely or become mobilized as a whole (Visnjic et al., 2004; Zhu et al., 2007; Méndez-Ferrer et al., 2010; Omatsu et al., 2010). This suggests either that the depletion systems are inefficient or that there is some redundancy in the system (or both). It further raises the possibility that more than one niche environment exists, and the distinct niches may house different subtypes of HSCs. In favor of this concept, several groups have identified long-term quiescent, so-called dormant HSCs in the healthy adult bone marrow. These cells harbor the highest self-renewal capacity among all blood progenitors and, although typically dormant during homeostasis, they can be induced to reenter the cell cycle in response to cytokines like type I or II IFN, G-CSF, or injury signals such as those generated by chemotherapy-induced myelosuppression (Wilson et al., 2008; Essers et al., 2009; Foudi et al., 2009; Schaniel and Moore, 2009; Baldridge et al., 2010; Trumpp et al., 2010; Takizawa et al., 2011). Thus, we postulate the existence of two highly similar niches, one harboring dormant HSCs and one housing self-renewing HSCs (Fig. 2; Trumpp et al., 2010). Both niches may rely on perivascular MSCs, but the endosteal niche could maintain a small dormant HSC population via the additional presence of TPO-producing OBs embedded in a calcium-rich microenvironment. Because CAR cells promote HSC self-renewing division, they are likely only part of the perivascular niche. In the remainder of this review, we will use this model as a basis to integrate recent studies demonstrating that monocytes/macrophages are another niche cell type that is critical for HSC maintenance during homeostasis and G-CSF–induced HSC mobilization. Figure 2. Model illustrating the quiescent endosteal and the active perivascular HSC niche during bone marrow homeostasis. Deeply quiescent (dormant) HSCs in the endosteal niche are likely in close contact with OBs and nestin+ MSCs, both of which supply HSC maintenance and quiescence factors, including CXCL12, SCF, Ang-1, VCAM-1, and TPO, and cooperate to retain HSCs in their niche. MSCs can generate OBs, adipocytes, and chondrocytes. The perivascular niche is more distant from the endosteum and does not contain OBs, but includes perivascular CAR cells that secrete factors that promote self-renewal of active HSCs, which are significantly more abundant than dormant HSCs. Self-renewal is also stimulated by Notch ligands expressed by sinusoidal endothelial cells. Both niches contain perivascular nestin+ MSCs as a key component. Different subtypes of phagocytes support the maintenance of OBs (osteomacs) and maintenance and proliferation of MSCs (macrophages). They also induce the expression of HSC maintenance factors. The SNS inhibits MSC proliferation and induces circadian oscillations of CXCL12 expression. CAMS, cell adhesion molecules. Macrophages join the niche Bone marrow niches are highly complex, and three recent studies (including Chow et al. and Christopher et al. in the previous issue of the JEM) add to this complexity by reporting a role for bone marrow mononuclear phagocytes in promoting maintenance and retention of HSCs (Winkler et al., 2010). First, Chow et al. (2011) developed a strategy to subdivide neutrophils, Gr-1hi and Gr-1lo monocytes, and Gr1−F4/80+CD169+ macrophages. Next, they used four different models to deplete various overlapping monocyte/macrophage populations from mouse bone marrow. These included clodronate-loaded liposomes, an inducible c-fms promoter-driven Fas-mediated cell depletion mouse model (macrophage Fas-induced apoptosis [MAFIA]), and transgenic animals in which the DTR is driven by Gr-1 or CD169. In all cases, loss of monocytes and/or macrophages was associated with mobilization of HSCs out of the bone marrow into the peripheral blood and spleen (Fig. 3). This was associated with a 40% reduction in CXCL12 protein in the bone marrow extracellular fluid. Because CXCL12-mediated activation of the CXCR4 receptor on HSCs is a critical niche retention signal (Lapidot and Petit, 2002; Broxmeyer, 2008), these data provide a plausible explanation for the phenotype observed in these mice. Mobilization was similar in all depletion models. Thus, as the CD169-driven DTR model was the most restricted, the loss of CD169+ macrophages rather than monocytes appears to be critical for the mobilization of HSCs (Chow et al., 2011). Figure 3. Model illustrating the quiescent endosteal niche and the active perivascular HSC niche after stimulation with G-CSF or depletion of monocytes/macrophages. Upon stimulation with G-CSF, which binds G-CSFR on monocytic cells, the monocytes/macrophages disappear. As a consequence of their missing supportive activity, OB activity is decreased and nestin+ MSCs no longer express high levels of SCF, VCAM1, Ang-1, and CXCL12. Because CXCL12-mediated activation of the CXCR4 receptor on HSCs is a critical niche retention signal, HSCs get mobilized into the periphery via entry into the sinusoids. Alternatively, the CXCR4/CXCL12 axis can be inhibited by the clinically used mobilizing agent AMD3100. Additionally, G-CSF stimulates the SNS, which contributes to HSC mobilization. CAMS, cell adhesion molecules. To further address the mechanism of this striking mobilization effect, the authors isolated nestin+ MSCs and OBs from clodronate-treated and control mice and examined the expression of HSC maintenance factors in the presence or absence of mononuclear phagocytes. Although the expression of CXCL12, SCF, Ang-1, and VCAM1 mRNAs were strongly reduced in nestin+ MSCs in the absence of phagocytes, they were unchanged in sorted OBs. Phagocyte depletion did not reduce the numbers of either cell population. These data suggest that macrophages are positive regulators of the nestin+ MSC niche cells that are required to maintain expression of various HSC retention factors, including CXCL12. But how do macrophages talk to MSCs? Although the authors provide data suggesting that this effect is mediated by a protein secreted by macrophages, and they rule out a role for IGF-1, IL-1, TNF, and IL-10, the candidate factors remain elusive. Thus, the identification of the proteins that mediate the positive interaction between CD169+ macrophages and nestin+ MSCs remains a key issue for future studies. A critical role for monocytes/macrophages was also suggested by Winkler et al. (2010). After phagocyte depletion using clodronate liposomes or the MAFIA model, they also observed mobilization of functional HSCs with repopulating activity. This phenotype was associated with a decrease in transcripts of CXCL12, Ang-1, and SCF in total bone marrow and in endosteal stroma, which likely contains OBs and MSCs. Most strikingly, clodronate depletion was associated with the loss of F4/80+ macrophages specifically associated with the endosteal lining, which have previously been characterized as osteomacs (Chang et al., 2008; Winkler et al., 2010). The loss of phagocytes caused a significant decrease in osteoblastic activity at the bone surface, as the proportion of bone surface lined with OBs and the amount of newly formed bone matrix decreased. The loss of OBs was independently confirmed by the decrease of osteocalcin expression in the bone marrow. The latter data conflict with the study by Chow et al. (2011), who did not observe reduced OB numbers or decreased OB expression of HSC maintenance factors when phagocytes were depleted. Although this issue has yet to be resolved, the data from both studies suggest that phagocytes, potentially of different types, are likely to form part of both niches (Fig. 2) and are positive regulators of perivascular MSCs and endosteal OBs. G-CSF induces HSC mobilization by targeting monocytes The recent studies also addressed the mechanism of G-CSF–induced mobilization of HSCs into the peripheral blood (Winkler et al., 2010; Chow et al., 2011; Christopher et al., 2011). This method has been used for many years to harvest functional HSCs for stem cell transplantation (Gertz, 2010), but the cellular and molecular mechanisms for this striking indirect effect (HSCs do not express G-CSF receptor) remain poorly understood. Christopher et al. (2011) show that G-CSF receptor expression on cells of the monocytic lineage is sufficient for G-CSF–induced mobilization of HSCs (Fig. 3). By generating mice expressing G-CSFR exclusively on CD68+ monocytic cells, they show that HSC mobilization in these mice was restored compared with mice lacking the receptor, suggesting that activation of the G-CSFR in mononuclear phagocytes is sufficient to mediate HSPC mobilization. This may settle a long-standing debate about the role of neutrophils and neutrophil-derived proteases (neutrophil elastase, cathepsin G, and MMP9), which were previously thought to be the main drivers of HSC mobilization in response to G-CSF (Lévesque et al., 2001; Heissig et al., 2002; Petit et al., 2002; Christopherson et al., 2003). Although not excluding a contribution of neutrophils to G-CSF–induced mobilization, Christopher et al. (2011) demonstrate that monocytic cells are the key player in this process, at least in mice. The data by Christopher et al. (2011) suggest that G-CSF–stimulated monocytes cause the mobilization of HSCs out of their niche, but how does this work? Mobilization of HSCs from bone marrow niches has been generally attributed to an overall decrease of bone marrow and serum CXCL12 levels, which activates the CXCR4 receptor to promote HSC retention—an interaction that is blocked by the mobilizing compound AMD3100 (Broxmeyer et al., 2005; De Clercq, 2009). Macrophages have now been identified as niche components that positively regulate MSCs and OBs, which in turn provide HSCs with the necessary extracellular retention factors, including CXCL12. Are these interactions disrupted by G-CSF? Christopher et al. (2011) use flow cytometry to show that inflammatory and resident monocytes were reduced up to fivefold 3 d after G-CSF treatment. Although it seems unlikely that these two monocyte populations also contain macrophages, the study by Winkler et al. (2010) showed that F4/80+ osteomacs, which form a structure similar to a canopy around endosteal OBs, were undetectable as early as 2 d after G-CSF stimulation, returning 4 d after cessation of G-CSF treatment. Because the G-CSF–induced loss of monocytes/macrophages is similar to the situation in the aforementioned phagocyte depletion models, it is not surprising that G-CSF treatment resulted in the rapid depletion of endosteal osteoblastic activity (Winkler et al., 2010). Histomorphometric methods revealed a 90% reduction of the bone formation rate, specifically at the endosteum. Moreover, the proportion of bone surface lined with OBs or newly formed bone matrix was decreased by >16-fold during mobilization. This effect was apparent within 2 d of G-CSF treatment, preceding the appearance of HSCs in the blood (Winkler et al., 2010). As OBs themselves do not express the G-CSFR, this effect is indirect and likely to be a consequence of G-CSF–mediated depletion of monocytes/macrophages. A positive effect of macrophages on OBs was further supported by data obtained in co-culture studies suggesting that macrophages produce factors that support the growth, survival, and mineralization of OBs (Chang et al., 2008; Christopher et al., 2011). In addition to the effect on OBs, G-CSF–induced phagocyte depletion also negatively impacts the function of nestin+ MSCs within the HSC niche. First, phagocyte depletion inhibited the proliferation of MSCs and their expression of osteoblastic differentiation genes such as osteoglycin, bone morphogenetic protein 4, and adiponectin (Méndez-Ferrer et al., 2010). The expression of transcripts encoding HSC maintenance factors including Ang-1, SCF, and VCAM1 by MSCs also decreased. Most importantly, the expression of CXCL12, which is not produced by macrophages themselves, decreased by approximately fivefold in nestin+ MSCs in response to G-CSF stimulation (Méndez-Ferrer et al., 2010). Finally, although HSC mobilization induced by phagocyte depletion and via G-CSF stimulation appear to be related, the latter method is up to 10 times more efficient, indicating that G-CSF may also act on a different cell type. Along these lines, Chow et al. showed that the SNS is a possible macrophage-independent G-CSF target (Fig. 3). SNS fibers are intermingled with nestin+ MSCs and inhibit HSC retention by negatively affecting nestin+ MSC activity (Katayama et al., 2006; Méndez-Ferrer et al., 2008, 2010). The G-CSF–mediated increase in sympathetic tone promotes progenitor cell egress, and thus represents a macrophage/monocyte-independent pathway for G-CSF–mediated mobilization. This provides some explanation as to why G-CSF is a much more effective HSC mobilizer than is phagocyte depletion (Chow et al., 2011). The HSC niche unit These recent studies have significantly increased our understanding of the cellular components comprising the HSC niche, and they provide solid data for the involvement of nestin+ MSCs, CAR cells, and macrophages. The finding that macrophages control both MSCs and OBs, and the observation that MSCs themselves generate OBs, suggest a complex network of cell–cell interactions forming the three dimensional structure of the HSC niche unit. In a fourth dimension, this unit is likely to constantly adapt to various in vivo challenges to maintain HSCs during steady state and under stress. Nevertheless, several questions remain to be answered. First, the relationship between nestin+ MCSs and CAR cells needs to be dissected. In addition, although macrophages seem likely to be the major phagocyte player, different methods and only partially overlapping markers have been used to identify monocytes and macrophages in the marrow and osteomacs at the endosteum. Thus, it will be interesting to see whether several different types of phagocytes are niche components or whether only specific subtypes are functionally involved. The macrophage-derived signaling molecules that positively influence nestin+ MSCs and OBs also remain elusive. Moreover, it remains to be demonstrated which cell types directly contact HSCs within the niche and which cells produce soluble signaling molecules and thus act exclusively in a paracrine manner. There is also significant disagreement about the role of OBs, whether (and to what level) they express HSC maintenance factors (i.e., Ang-1, CXCL12, and SCF), and whether their expression is altered in response to G-CSF. Moreover, it is likely that yet other cell types may contribute to HSC niche function. For example, sinusoidal endothelial cells have been suggested to play a role, as they produce angiocrine factors such as Notch ligands, which promote HSC self-renewal and regeneration after bone marrow injury (Butler et al., 2010; Kobayashi et al., 2010). Whether sinusoidal endothelial cells also contribute to G-CSF–mediated mobilization remains to be addressed. A recent study suggested that adipocytes, another cell type produced by MSCs, have an adverse effect on HSC maintenance (Naveiras et al., 2009). Finally, models suggesting the existence of two distinct HSC niches in the bone marrow remain to be experimentally confirmed (Fig. 2; Trumpp et al., 2010). Although such a model would incorporate the currently available data about the niche components and link it to the finding that dormant and actively self-renewing HSCs coexist in the marrow, it remains very difficult to identify and discriminate these subpopulations in situ, as no definitive markers have been reported so far. Future studies will also need to further explore the different cocktails of secreted and membrane-bound signaling molecules and adhesion receptors expressed by the various niche components that control HSC dormancy, self-renewal, lineage-specific priming, and survival of HSCs during steady-state hematopoiesis and in response to stress. Concluding remarks The bone marrow HSC niche has grown up, and it appears as complex as one would anticipate considering the various functions that HSCs have to fulfill during development, homeostasis, and in response to stress situations, such as chemotherapy-induced cytopenia or infection with bacteria or viruses. Although we now seem to know some, and perhaps the main, cellular players, we are far from understanding the three-dimensional architecture of the HSC niche during homeostasis or how the niche controls HSC function during stress. Our increased understanding of normal HSC niches will foster studies on the leukemic HSC nichsecbe, which may reveal novel strategies for targeting therapy-resistant leukemic stem cells in hematopoietic malignancies.