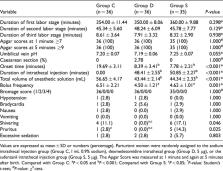
Epidural analgesia is a central nerve block technique achieved by injection of a local anaesthetic close to the nerves that transmit pain, and is widely used as a form of pain relief in labour. However, there are concerns about unintended adverse effects on the mother and infant. This is an update of an existing Cochrane Review ( Epidural versus non‐epidural or no analgesia in labour ), last published in 2011. To assess the effectiveness and safety of all types of epidural analgesia, including combined‐spinal‐epidural (CSE) on the mother and the baby, when compared with non‐epidural or no pain relief during labour. We searched Cochrane Pregnancy and Childbirth’s Trials Register ( ClinicalTrials.gov ), the WHO International Clinical Trials Registry Platform ( ICTRP ) (30 April 2017), and reference lists of retrieved studies. Randomised controlled trials comparing all types of epidural with any form of pain relief not involving regional blockade, or no pain relief in labour. We have not included cluster‐randomised or quasi‐randomised trials in this update. Two review authors independently assessed trials for inclusion and risks of bias, extracted data and checked them for accuracy. We assessed selected outcomes using the GRADE approach. Fifty‐two trials met the inclusion criteria and we have included data from 40 trials, involving over 11,000 women. Four trials included more than two arms. Thirty‐four trials compared epidural with opioids, seven compared epidural with no analgesia, one trial compared epidural with acu‐stimulation, one trial compared epidural with inhaled analgesia, and one trial compared epidural with continuous midwifery support and other analgesia. Risks of bias varied throughout the included studies; six out of 40 studies were at high or unclear risk of bias for every bias domain, while most studies were at high or unclear risk of detection bias. Quality of the evidence assessed using GRADE ranged from moderate to low quality. Pain intensity as measured using pain scores was lower in women with epidural analgesia when compared to women who received opioids (standardised mean difference ‐2.64, 95% confidence interval (CI) ‐4.56 to ‐0.73; 1133 women; studies = 5; I 2 = 98%; low‐quality evidence) and a higher proportion were satisfied with their pain relief, reporting it to be "excellent or very good" (average risk ratio (RR) 1.47, 95% CI 1.03 to 2.08; 1911 women; studies = 7; I 2 = 97%; low‐quality evidence). There was substantial statistical heterogeneity in both these outcomes. There was a substantial decrease in the need for additional pain relief in women receiving epidural analgesia compared with opioid analgesia (average RR 0.10, 95% CI 0.04 to 0.25; 5099 women; studies = 16; I 2 = 73%; Tau 2 = 1.89; Chi 2 = 52.07 (P < 0.00001)). More women in the epidural group experienced assisted vaginal birth (RR 1.44, 95% CI 1.29 to 1.60; 9948 women; studies = 30; low‐quality evidence). A post hoc subgroup analysis of trials conducted after 2005 showed that this effect is negated when trials before 2005 are excluded from this analysis (RR 1.19, 95% CI 0.97 to 1.46). There was no difference between caesarean section rates (RR 1.07, 95% CI 0.96 to 1.18; 10,350 women; studies = 33; moderate‐quality evidence), and maternal long‐term backache (RR 1.00, 95% CI 0.89 to 1.12; 814 women; studies = 2; moderate‐quality evidence). There were also no clear differences between groups for the neonatal outcomes, admission to neonatal intensive care unit (RR 1.03, 95% CI 0.95 to 1.12; 4488 babies; studies = 8; moderate‐quality evidence) and Apgar score less than seven at five minutes (RR 0.73, 95% CI 0.52 to 1.02; 8752 babies; studies = 22; low‐quality evidence). We downgraded the evidence for study design limitations, inconsistency, imprecision in effect estimates, and possible publication bias. Side effects were reported in both epidural and opioid groups. Women with epidural experienced more hypotension, motor blockade, fever, and urinary retention. They also had longer first and second stages of labour, and were more likely to have oxytocin augmentation than the women in the opioid group. Women receiving epidurals had less risk of respiratory depression requiring oxygen, and were less likely to experience nausea and vomiting than women receiving opioids. Babies born to women in the epidural group were less likely to have received naloxone. There was no clear difference between groups for postnatal depression, headache, itching, shivering, or drowsiness. Maternal morbidity and long‐term neonatal outcomes were not reported. Epidural analgesia resulted in less reported pain when compared with placebo or no treatment, and with acu‐stimulation. Pain intensity was not reported in the trials that compared epidural with inhaled analgesia, or continuous support. Few trials reported on serious maternal side effects. Low‐quality evidence shows that epidural analgesia may be more effective in reducing pain during labour and increasing maternal satisfaction with pain relief than non‐epidural methods. Although overall there appears to be an increase in assisted vaginal birth when women have epidural analgesia, a post hoc subgroup analysis showed this effect is not seen in recent studies (after 2005), suggesting that modern approaches to epidural analgesia in labour do not affect this outcome. Epidural analgesia had no impact on the risk of caesarean section or long‐term backache, and did not appear to have an immediate effect on neonatal status as determined by Apgar scores or in admissions to neonatal intensive care. Further research may be helpful to evaluate rare but potentially severe adverse effects of epidural analgesia and non‐epidural analgesia on women in labour and long‐term neonatal outcomes. Epidurals for pain relief in labour What is the issue? We set out to assess the effectiveness of all kinds of epidural analgesia (including combined‐spinal‐epidural) on the mother and the baby, when compared with non‐epidural or no pain relief during labour. Why is this important? Pain relief is important for women in labour. Pharmacological methods of pain relief include breathing in of nitrous oxide, injection of opioids and local analgesia with an epidural for a central nerve block. Epidurals are widely used for pain relief in labour and involve an injection of a local anaesthetic into the lower region of the back close to the nerves that transmit pain. Epidural solutions are given by bolus injection (a large, rapid injection), continuous infusion or using a patient‐controlled pump. Lower concentrations of local anaesthetic when given together with an opiate allow women to maintain the ability to move around during labour and to actively participate in the birth. Combined‐spinal‐epidural involves a single injection of local anaesthetic or opiate into the cerebral spinal fluid for fast onset of pain relief, as well as insertion of the epidural catheter for continuing pain relief. Side effects such as itchiness, drowsiness, shivering and fever have been reported. Rare but potentially severe adverse effects of epidural analgesia can occur, such as severe long‐lasting headache after the injection, or nerve injury. What evidence did we find? We searched for evidence in April 2017 and identified 40 trials, involving over 11,000 women, that contributed information to this review. The trials varied in the quality of their methods. All but six studies compared epidural analgesia with injected opioid drugs. Epidurals may relieve labour pain more effectively than opioids, and more women may be more satisfied with epidural as pain relief. Overall, women using epidural analgesia may be more likely to require forceps or ventouse to assist with the birth when compared with opioid drugs. However we did not see this effect in studies conducted since 2005, where the use of lower concentrations of local anaesthetic and more modern epidural techniques such as patient‐controlled epidural analgesia (PCEA) were more likely. Epidural in comparison to opioids probably makes little or no difference to caesarean section rates, women with long‐term backache, effects on the baby at birth or the number of babies who were admitted to neonatal intensive care. Women who used epidurals can have problems passing urine and can suffer fever. There are highly variable findings such as a longer labour, experiencing very low blood pressure, and being unable to move for a period of time after the birth (motor blockade), probably due to higher concentrations of local anaesthetic being used in the epidural or the use of epidural infusions rather than epidural doses of pain relief administered at intervals. However, women who received opioid drugs also showed some side effects such as a slowing of their breathing so that they needed to wear an oxygen mask, and more nausea and vomiting. More babies whose mothers received opioids were given a drug to counteract the effects of the opioids. There was no difference between women in the epidural or opioid groups for postnatal depression, headaches, itching, shivering, or drowsiness. Women with epidurals reported less pain compared to women with placebo or no treatment, or acu‐stimulation. Pain was not reported in the trials that compared epidural with inhaled analgesia, or continuous support. What does this mean? Epidurals may reduce pain during labour more effectively than any other form of pain relief, and may increase maternal satisfaction with pain relief. However, some women who have an epidural instead of opioid drugs may be more likely to have an assisted vaginal birth, but this finding probably reflects the higher concentrations of local anaesthetics used traditionally rather than the low concentrations of modern epidurals. Further research would be helpful, using more consistent measures of reducing the adverse outcomes with epidurals.