- Record: found
- Abstract: found
- Article: found
Effects of Sacubitril/Valsartan on Serum Lipids in Heart Failure With Preserved Ejection Fraction
Read this article at
Abstract
Background
Dyslipidemia is common in heart failure with preserved ejection fraction. Sacubitril/valsartan improves glycemic control and augments natriuretic peptide signaling, providing mechanisms by which sacubitril/valsartan may affect serum lipids. However, empiric data on these effects are lacking.
Methods and Results
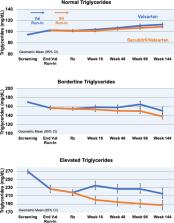
We analyzed 4774 participants from PARAGON‐HF (Prospective Comparison of Angiotensin Receptor–Neprilysin Inhibitor With Angiotensin‐Receptor Blockers Global Outcomes in Heart Failure With Preserved Ejection Fraction) with available screening lipids. During follow‐up visits, we analyzed the treatment effect on lipid levels and assessed for interaction by baseline lipid levels. At the 16‐week visit, we adjusted these treatment effects for the change in several biomarkers (including hemoglobin A 1c and urinary cyclic guanosine monophosphate/creatinine [a biomarker of natriuretic peptide activation]). The average age was 73±8 years, 52% were women, 43% had diabetes mellitus, and 64% were on statin therapy. Compared with valsartan, sacubitril/valsartan reduced triglycerides −5.0% (95% CI, −6.6% to −3.5%), increased high‐density lipoprotein cholesterol +2.6% (95% CI, +1.7% to +3.4%), and increased low‐density lipoprotein cholesterol +1.7% (95% CI, +0.4% to +3.0%). Sacubitril/valsartan reduced triglycerides most among those with elevated baseline levels (triglycerides≥200 mg/dL) ( P‐interaction<0.001), and at 16 weeks by −13.0% (95% CI, −18.1% to −7.6%), or −29.9 (95% CI, −44.3 to −15.5) mg/dL, in this group. Adjusting for the change in urinary cyclic guanosine monophosphate/creatinine significantly attenuated treatment effects on triglycerides and high‐density lipoprotein cholesterol, but not low‐density lipoprotein cholesterol, while adjusting for other biomarkers did not significantly alter the treatment effects.
Conclusions
Sacubitril/valsartan significantly reduces triglycerides compared with valsartan, an effect that was nearly threefold stronger in those with elevated baseline triglycerides. Modest increases in high‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol cholesterol were also observed with therapy. The underlying mechanism(s) of changes in high‐density lipoprotein cholesterol and triglycerides are related to sacubitril/valsartan’s effects on natriuretic peptide activity.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome
- Record: found
- Abstract: found
- Article: not found
Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia
- Record: found
- Abstract: found
- Article: not found