- Record: found
- Abstract: found
- Article: found
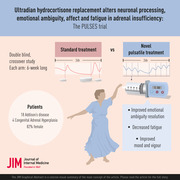
Ultradian hydrocortisone replacement alters neuronal processing, emotional ambiguity, affect and fatigue in adrenal insufficiency: The PULSES trial

Read this article at
Abstract
Background
Primary adrenal insufficiency (PAI) mortality and morbidity remain unacceptably high, possibly arising as glucocorticoid replacement does not replicate natural physiology. A pulsatile subcutaneous pump can closely replicate cortisol's circadian and ultradian rhythm.
Objectives
To assess the effect of pump therapy on quality of life, mood, functional neuroimaging, behavioural/cognitive responses, sleep and metabolism.
Methods
A 6‐week randomised, crossover, double‐blinded and placebo‐controlled feasibility study of usual dose hydrocortisone in PAI administered as either pulsed subcutaneous or standard care in Bristol, United Kingdom (ISRCTN67193733). Participants were stratified by adrenal insufficiency type. All participants who received study drugs are included in the analysis. The primary outcome, the facial expression recognition task (FERT), occurred at week 6.
Results
Between December 2014 and 2017, 22 participants were recruited – 20 completed both arms, and 21 were analysed. The pump was well‐tolerated. No change was seen in the FERT primary outcome; however, there were subjective improvements in fatigue and mood. Additionally, functional magnetic resonance imaging revealed differential neural processing to emotional cues and visual stimulation. Region of interest analysis identified the left amygdala and insula, key glucocorticoid‐sensitive regions involved in emotional ambiguity. FERT post hoc analysis confirmed this response. There were four serious adverse events (AE): three intercurrent illnesses requiring hospitalisation (1/3, 33.3% pump) and a planned procedure (1/1, 100% pump). There was a small number of expected AEs: infusion site bruising/itching (3/5, 60% pump), intercurrent illness requiring extra (3/7, 42% pump) and no extra (4/6, 66% pump) steroid.
Abstract
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research
- Record: found
- Abstract: found
- Article: not found
Development and validation of brief measures of positive and negative affect: the PANAS scales.
- Record: found
- Abstract: found
- Article: not found