- Record: found
- Abstract: found
- Article: found
Cerebral vasomotor reactivity: steady-state versus transient changes in carbon dioxide tension

Read this article at
Abstract
New Findings
-
What is the central question of this study?
The relationship between changes in cerebral blood flow and arterial carbon dioxide tension is used to assess cerebrovascular function. Hypercapnia is generally evoked by two methods, i.e. steady-state and transient increases in carbon dioxide tension. In some cases, the hypercapnia is immediately preceded by a period of hypocapnia. It is unknown whether the cerebrovascular response differs between these methods and whether a period of hypocapnia blunts the subsequent response to hypercapnia.
-
What is the main finding and its importance?
The cerebrovascular response is similar between steady-state and transient hypercapnia. However, hyperventilation-induced hypocapnia attenuates the cerebral vasodilatory responses during a subsequent period of rebreathing-induced hypercapnia.
Cerebral vasomotor reactivity (CVMR) to changes in arterial carbon dioxide tension
(
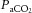 ) is assessed during steady-state or transient changes in
) is assessed during steady-state or transient changes in
 . This study tested the following two hypotheses: (i) that CVMR during steady-state
changes differs from that during transient changes in
. This study tested the following two hypotheses: (i) that CVMR during steady-state
changes differs from that during transient changes in
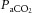 ; and (ii) that CVMR during rebreathing-induced hypercapnia would be blunted when
preceded by a period of hyperventilation. For each hypothesis, end-tidal carbon dioxide
tension (
; and (ii) that CVMR during rebreathing-induced hypercapnia would be blunted when
preceded by a period of hyperventilation. For each hypothesis, end-tidal carbon dioxide
tension (
 ) middle cerebral artery blood velocity (CBFV), cerebrovascular conductance index
(CVCI; CBFV/mean arterial pressure) and CVMR (slope of the linear regression between
changes in CBFV and CVCI
versus
) middle cerebral artery blood velocity (CBFV), cerebrovascular conductance index
(CVCI; CBFV/mean arterial pressure) and CVMR (slope of the linear regression between
changes in CBFV and CVCI
versus
 ) were assessed in eight individuals. To address the first hypothesis, measurements
were made during the following two conditions (randomized): (i) steady-state increases
in
) were assessed in eight individuals. To address the first hypothesis, measurements
were made during the following two conditions (randomized): (i) steady-state increases
in
 of 5 and 10 Torr above baseline; and (ii) rebreathing-induced transient breath-by-breath
increases in
of 5 and 10 Torr above baseline; and (ii) rebreathing-induced transient breath-by-breath
increases in
 . The linear regression for CBFV
versus
. The linear regression for CBFV
versus
 (
P = 0.65) and CVCI
versus
(
P = 0.65) and CVCI
versus
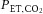 (
P = 0.44) was similar between methods; however, individual variability in CBFV or CVCI
responses existed among subjects. To address the second hypothesis, the same measurements
were made during the following two conditions (randomized): (i) immediately following
a brief period of hypocapnia induced by hyperventilation for 1 min followed by rebreathing;
and (ii) during rebreathing only. The slope of the linear regression for CBFV
versus
(
P = 0.44) was similar between methods; however, individual variability in CBFV or CVCI
responses existed among subjects. To address the second hypothesis, the same measurements
were made during the following two conditions (randomized): (i) immediately following
a brief period of hypocapnia induced by hyperventilation for 1 min followed by rebreathing;
and (ii) during rebreathing only. The slope of the linear regression for CBFV
versus
 (
P < 0.01) and CVCI
versus
(
P < 0.01) and CVCI
versus
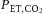 (
P < 0.01) was reduced during hyperventilation plus rebreathing relative to rebreathing
only. These results indicate that cerebral vasomotor reactivity to changes in
(
P < 0.01) was reduced during hyperventilation plus rebreathing relative to rebreathing
only. These results indicate that cerebral vasomotor reactivity to changes in
 is similar regardless of the employed methodology to induce changes in
is similar regardless of the employed methodology to induce changes in
 and that hyperventilation-induced hypocapnia attenuates the cerebral vasodilatory
responses during a subsequent period of rebreathing-induced hypercapnia.
and that hyperventilation-induced hypocapnia attenuates the cerebral vasodilatory
responses during a subsequent period of rebreathing-induced hypercapnia.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Regional brain blood flow in man during acute changes in arterial blood gases.
- Record: found
- Abstract: found
- Article: not found
Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation.
- Record: found
- Abstract: found
- Article: not found