- Record: found
- Abstract: found
- Article: not found
Research progress in methods for detecting neutralizing antibodies against SARS-CoV-2
Read this article at
Abstract
The emergence of SARS-CoV-2 has seriously affected the lives of people worldwide. Clarifying the attenuation rule of SARS-CoV-2 neutralizing antibody (NAb) in vivo is the key to prevent reinfection and recurrence of virus. Currently, the commonly used methods for detecting NAb include virus neutralization tests, pseudovirus neutralization assays, lateral flow immunochromatography and enzyme-linked immunosorbent assays. The detection of NAb not only can be used to evaluate the level of immunity after vaccination or infection but also can provide important theoretical support for virus reinfection, recurrence and vaccine iteration. In this research, the related technologies of SARS-CoV-2 NAb detection were reviewed, aiming to provide better research ideas for SARS-CoV-2 epidemic prevention and control.
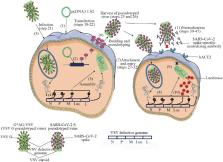
Graphical abstract
SARS-CoV-2 neutralizing antibody (NAb) in vivo is the key to prevent reinfection and recurrence of virus. Currently, the commonly used methods for detecting NAb include virus neutralization tests, pseudovirus neutralization assays, lateral flow im-munochromatography and enzyme-linked immunosorbent assays. The detection of NAb not only be used to evaluate the level of immunity after vaccination or infection but also can provide important theoretical support for virus reinfection, recurrence and vaccine iteration.
Related collections
Most cited references99
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor

- Record: found
- Abstract: found
- Article: found
Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation
- Record: found
- Abstract: found
- Article: not found

