- Record: found
- Abstract: found
- Article: found
Extended-release ketamine tablets for treatment-resistant depression: a randomized placebo-controlled phase 2 trial
Read this article at
Abstract
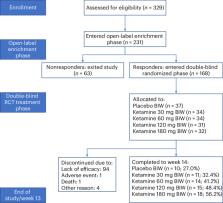
Ketamine has rapid-onset antidepressant activity in patients with treatment-resistant major depression (TRD). The safety and tolerability of racemic ketamine may be improved if given orally, as an extended-release tablet (R-107), compared with other routes of administration. In this phase 2 multicenter clinical trial, male and female adult patients with TRD and Montgomery–Asberg Depression Rating Scale (MADRS) scores ≥20 received open-label R-107 tablets 120 mg per day for 5 days and were assessed on day 8 (enrichment phase). On day 8, responders (MADRS scores ≤12 and reduction ≥50%) were randomized on a 1:1:1:1:1 basis to receive double-blind R-107 doses of 30, 60, 120 or 180 mg, or placebo, twice weekly for a further 12 weeks. Nonresponders on day 8 exited the study. The primary endpoint was least square mean change in MADRS for each active treatment compared with placebo at 13 weeks, starting with the 180 mg dose, using a fixed sequence step-down closed test procedure. Between May 2019 and August 2021, 329 individuals were screened for eligibility, 231 entered the open-label enrichment phase (days 1–8) and 168 responders were randomized to double-blind treatment. The primary objective was met; the least square mean difference of MADRS score for the 180 mg tablet group and placebo was −6.1 (95% confidence interval 1.0 to 11.16, P = 0.019) at 13 weeks. Relapse rates during double-blind treatment showed a dose response from 70.6% for placebo to 42.9% for 180 mg. Tolerability was excellent, with no changes in blood pressure, minimal reports of sedation and minimal dissociation. The most common adverse events were headache, dizziness and anxiety. During the randomized phase of the study, most patient dosing occurred at home. R-107 tablets were effective, safe and well tolerated in a patient population with TRD, enriched for initial response to R-107 tablets. ClinicalTrials.gov registration: ACTRN12618001042235.
Abstract
A phase 2 dose-finding study of an extended-release tablet formulation of ketamine in patients with treatment-resistant depression shows that this formulation overcomes many of the limitations associated with the use of intravenous or intranasal ketamine formulations.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
A new depression scale designed to be sensitive to change
- Record: found
- Abstract: found
- Article: not found
The clinical global impressions scale: applying a research tool in clinical practice.
- Record: found
- Abstract: found
- Article: not found