- Record: found
- Abstract: found
- Article: found
COVID-19: more than a cytokine storm
letter
Giovanni Riva
1 ,
Vincenzo Nasillo
2 ,
Enrico Tagliafico
1 ,
Tommaso Trenti
1 ,
Patrizia Comoli
3 ,
Mario Luppi
2
,
4 September 2020
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Background
In these first months of coronavirus disease-19 (COVID-19) pandemic, a mainstream
pathogenetic hypothesis, likely stemming from early clinico-therapeutic observations,
has been suggesting that severe COVID-19 may represent a sort of hyperimmune disorder,
akin, in particular, to secondary hemophagocytic lymphohistiocytosis (sHLH) and macrophage
activation syndrome (MAS) [1–3]. In this view, COVID-19-associated cytokine storm,
with elevated plasma levels of IL-6, IL-1, and TNF-α, as well as ferritin and other
inflammatory biomarkers, has been considered as a typical sign of sHLH/MAS, but the
other “key feature” of COVID-19—the progressive lymphopenia with T cell exhaustion
[4–6]—has largely been neglected. Of note, both CD4+ and CD8+ T lymphocytes were found
to be remarkably decreased in severe cases (median 177.5 and 89.0 × 106/L, respectively),
when compared to moderate ones (median 381.5 and 254.0 × 106/L, respectively), thus
suggesting T cell lymphopenia may constitute a potential prognostic marker to be included
in the monitoring of COVID-19 patients [4]. Frequencies of IFN-γ-producing CD4+ T
cells (i.e., cytotoxic Th1 subset) tended to be lower in severe than in moderate illness
(median 14.1% versus 22.8%, respectively), possibly indicating a progressive skew
of the Th1/Th2 balance toward a tolerogenic response [4]. In addition, the percentages
of both memory Th cells and regulatory T cells were found to decrease in severe cases
[5].
Nonetheless, in patients with severe systemic hyper-inflammatory diseases driven by
other viral infections, hemophagocytic syndrome can be expected as a rare but life-threatening
event, and, indeed, sHLH has been recognized to occur in up to 4.3% of sepsis cases
[1]. Hence, in those COVID-19 patients showing massive hyperinflammation, a clinical
diagnosis of sHLH/MAS may be appropriate and deserves further investigation at the
histological level.
More recently, COVID-19 clinical syndrome and related immunopathogenesis have been
compared with sepsis, recalling the need to target the underlying and shared impairment
of protective T cell immunity, while suppressing the emergent cytokine storm [7–9].
In fact, severe COVID-19 has appeared as a peculiar clinicopathologic entity—yet poorly
understood from a mechanistic viewpoint—which however, by definition, may represent
a novel form of viral sepsis, being characterized by (a) T cell deficiencies, with
early and progressive lymphopenia; (b) systemic hyperinflammation, with a peculiar
time-course, often increasing at a late phase, when coagulopathy and fatal organ damage
may eventually occur; and (c) COVID-19-associated coagulopathy, displaying some unique
clinical and laboratory findings, compared with either disseminated intravascular
coagulation or sepsis-induced coagulopathy [10]. Further investigations are required
to shed light on the relationships between these clinic-immunologic features and organ
failure, possibly paving the way to the treatment (or even prevention) of severe COVID-19,
by modulation of host immune system with targeted immunotherapeutic drugs.
During the last few years, cancer immunotherapy with immune checkpoint inhibitors
(ICIs), such as anti-PD1/PD-L1 and anti-CTLA-4 monoclonal antibodies (e.g., nivolumab
and ipilimumab, respectively), has allowed impressive restoration of T cell immunity
against neoplastic cells, which commonly induce overexpression of PD-1/CTLA-4 ligands
to foster T cell exhaustion/anergy and break anti-tumor immune surveillance. Intriguingly,
several human viruses have been demonstrated to adopt such “cancer-like” immune-evasion
strategies, mainly by upregulation of PD-L1 in infected cells, in order to hamper
antiviral T cell responses and make a productive infection [11]. Recently, in the
attempt to improve antiviral T cell immunity in COVID-19 patients, clinical trials
have started to test such T cell activating treatments. Of note, an ongoing Spanish
phase 2 study (NCT04335305) seems the first to evaluate the attractive strategy of
combining anti-cytokine treatments with ICIs (namely, tocilizumab plus pembrolizumab).
Alongside monoclonal antibodies activating T lymphocytes, it has also been suggested
that the infusion of SARS-CoV-2-specific cytotoxic T lymphocytes, deriving from HLA-matched
convalescent donors, could be explored as innovative cell therapy for COVID-19 [12].
Actually, to maximize potential benefits of different immunotherapeutic approaches
against COVID-19, adequate patients’ selection is warranted, possibly performed on
the basis of putative biomarkers and immune profiles predictive of response. In addition,
by considering the typical disease course, often prolonged for several weeks, the
optimal timing for these treatments should be defined.
Thus, it seems conceivable that, during SARS-CoV-2 infection, especially in elderly
patients and less frequently in young people, something can go wrong at the delicate
interface between effective viral clearance and T cell tolerance. Indeed, COVID-19
may be characterized by different clinical pictures, ranging from almost asymptomatic/mild
infections in children and young individuals to lethal “sepsis-like” illness with
SARS, particularly in advanced age. What differs between these two distinct stages
of life, with regard to the antiviral response toward SARS-CoV-2 infection? Generally,
in young subjects and even more in children, T cell immunity is known to be more pronounced
and active, especially in terms of lymphocyte counts and adequate antiviral responses,
while aged individuals typically undergo a well-described decline in T cell functions,
which correlates with higher susceptibility to life-threatening infections, autoimmunity,
and cancer [13]. Susceptibility to SARS-CoV-2 infection, related to the different
functions and proportions of CD27dull and CD27bright memory B cells, throughout life,
has also recently been suggested [14].
In the fight against SARS-CoV-2 pandemic, a more comprehensive vision of COVID-19
immunopathogenesis and related clinical manifestations is warranted to reconcile COVID-19
hyper-inflammatory features—similarly observed in sepsis, sHLH/MAS, and cytokine release
syndrome (CRS) [3] induced by chimeric antigen receptor (CAR) T cell therapy, as well
as in Kaposi sarcoma herpesvirus-associated inflammatory cytokine syndrome (KICS)
[15] occurring in immunocompromised patients—with a renewed pivotal role played by
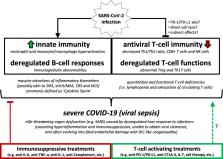
the impairment of antiviral T cell functions. In this perspective (Fig. 1), in parallel
with targeted immunosuppressive strategies, an effective reversal of T cell impairment
by immune-activating treatments should allow to improve viral clearance and promote
a better disease control with faster resolution, probably more akin to what naturally
occurs in children infected with SARS-CoV-2.
Fig. 1
Working model for COVID-19 immunopathogenesis and related immunomodulatory treatments.
Acronyms: SARS-CoV-2 severe acute respiratory syndrome-coronavirus-2, SARS severe
acute respiratory syndrome, SIRS severe inflammatory response syndrome, sHLH secondary
hemophagocytic lymphohistiocytosis, MAS macrophage activation syndrome, CRS cytokine
release syndrome, KICS Kaposi sarcoma herpesvirus-associated inflammatory cytokine
syndrome, COVID-19 coronavirus disease-19, DIC disseminated intravascular coagulation
Conclusions
SARS-COV-2 has arisen as a new pathogen frequently inducing sepsis-like manifestations
in the host. Indeed, based on actual evidence showing hyperinflammation as well as
T cell deficiencies and coagulation abnormalities, associated with life-threatening
organ dysfunction, severe COVID-19 may be well consistent with a clinical diagnosis
of viral sepsis, rather than with a mere hyper-inflammatory disease. This conceptual
framing may help to improve clinical management of severe COVID-19 patients, by providing
a rationale for the development of novel balanced immunomodulatory approaches, combining
both suppressive and activating immunotherapies.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: found
COVID-19: consider cytokine storm syndromes and immunosuppression
Puja Mehta, Daniel McAuley, Michael Brown … (2020)
- Record: found
- Abstract: found
- Article: not found
Clinical and immunologic features in severe and moderate Coronavirus Disease 2019
Guang Chen, Di Wu, Wei. Guo … (2020)
- Record: found
- Abstract: found
- Article: not found
Dysregulation of immune response in patients with COVID-19 in Wuhan, China
Chuan Qin, Luoqi Zhou, Ziwei Hu … (2020)