- Record: found
- Abstract: found
- Article: found
Systematic review of the economic evaluation model of assisted reproductive technology
Read this article at
Abstract
Background
With the increasing demand for fertility services, it is urgent to select the most cost-effective assisted reproductive technology (ART) treatment plan and include it in medical insurance. Economic evaluation reports are an important reference for medical insurance negotiation. The aim of this study is to systematically evaluate the economic evaluation research of ART, analyze the existing shortcomings, and provide a reference for the economic evaluation of ART.
Methods
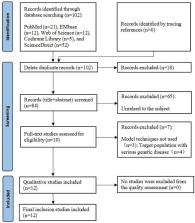
PubMed, EMbase, Web of Science, Cochrane Library and ScienceDirect databases were searched for relevant articles on the economic evaluation of ART. These articles were screened, and their quality was evaluated based on the Comprehensive Health Economics Evaluation Report Standard (CHEERS 2022), and the data on the basic characteristics, model characteristics and other aspects of the included studies were summarized.
Results
One hundred and two related articles were obtained in the preliminary search, but based on the inclusion criteria, 12 studies were used for the analysis, of which nine used the decision tree model. The model parameters were mainly derived from published literature and included retrospective clinical data of patients. Only two studies included direct non-medical and indirect costs in the cost measurement. Live birth rate was used as an outcome indicator in half of the studies.
Conclusion
Suggesting the setting of the threshold range in the field of fertility should be actively discussed, and the monetary value of each live birth is assumed to be in a certain range when the WTP threshold for fertility is uncertain. The range of the parameter sources should be expanded. Direct non-medical and indirect costs should be included in the calculation of costs, and the analysis should be carried out from the perspective of the whole society. In the evaluation of clinical effect, the effectiveness and safety indexes should be selected for a comprehensive evaluation, thereby making the evaluation more comprehensive and reliable. At least subgroup analysis based on age stratification should be considered in the relevant economic evaluation.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement
- Record: found
- Abstract: found
- Article: not found
Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations.
- Record: found
- Abstract: found
- Article: not found