INTRODUCTION
The global COVID-19 pandemic has resulted in > 770 million confirmed cases and > 6.9 million deaths as of 30 August 2023 [1]. A large number of COVID-19 convalescent patients has emerged over the past 3 y. The long-term impact of SARS-CoV-2 has become increasingly apparent and could become the next public health crisis, creating a heavy social and economic burden [2,3]. Thus, many countries and institutes are now focusing on the long-term consequences of COVID-19 [4–7]. Due to incomplete knowledge and evidence about the long-term effects of COVID-19 [8], many COVID-19 convalescent patients have not had access to adequate nursing instructions and rehabilitation guides [9]. Therefore, there is an urgent need to validate the health consequences among COVID-19 convalescent patients, which would provide a scientific basis for developing therapeutic strategies against COVID-19 in the long term.
Most existing studies have provided data pertaining to the health consequences of COVID-19 limited to 24 months post-infection, and the role of post-infection vaccination with respect to the long-term COVID-19 consequences has not been established. Previous studies have reported long COVID symptoms [10,11] among convalescent patients for 3 [12], 6 [5], 12 [6,13–16] and 18.5 months [17] post-infection. To date, the longest follow-up for COVID-19 convalescent patients is 24 months [7,18,19]. These studies indicated that the long COVID symptoms, routine laboratory tests, and radiographic abnormalities among COVID-19 convalescent patients improved over time, persistent symptoms, mental health disorders, and abnormalities in lung function tests were still common. We reported similar findings in a previous 18.5-month follow-up study [17]. It is unknown, however, if the abnormal conditions in these convalescent patients change over a longer duration of time. Moreover, previous studies have suggested that vaccination before SARS-CoV-2 infection reduced the risk of some symptoms, such as anxiety, depression, and fatigue [20,21]. Of note, in the real-world setting many people were vaccinated after being infected with COVID-19 [22,23]. Such convalescent patients have voiced concerns about how vaccination influences the health consequences of a previous infection. Indeed, few studies have depicted the role of post-infection vaccination with respect to long-term COVID-19 consequences [24]. A large retrospective analysis suggested that compared to individuals who were not vaccinated, individuals who received a single vaccine post-infection were less likely to develop long COVID after at least 20 weeks of infection [25]. Studies with a longer duration of follow-up and comprehensive analysis are urgently needed to validate the multiple health consequences of COVID-19 and establish the role of post-infection vaccination over the long-term.
In the current study we described the health consequences among COVID-19 convalescent patients 30 months post-infection, including long COVID symptoms, the 6-min walk test, the Borg dyspnea scale assessment, lung function testing, high-resolution computed tomography (HRCT) of the chest, anxiety, depression, insomnia, health-related quality of life, current working status, and biochemical indicators. We further determined the potential risk factors for long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We also comprehensively analyzed the impact of post-infection vaccination on the health consequences of COVID-19.
METHODS
Study design and participants
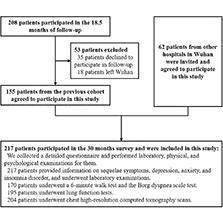
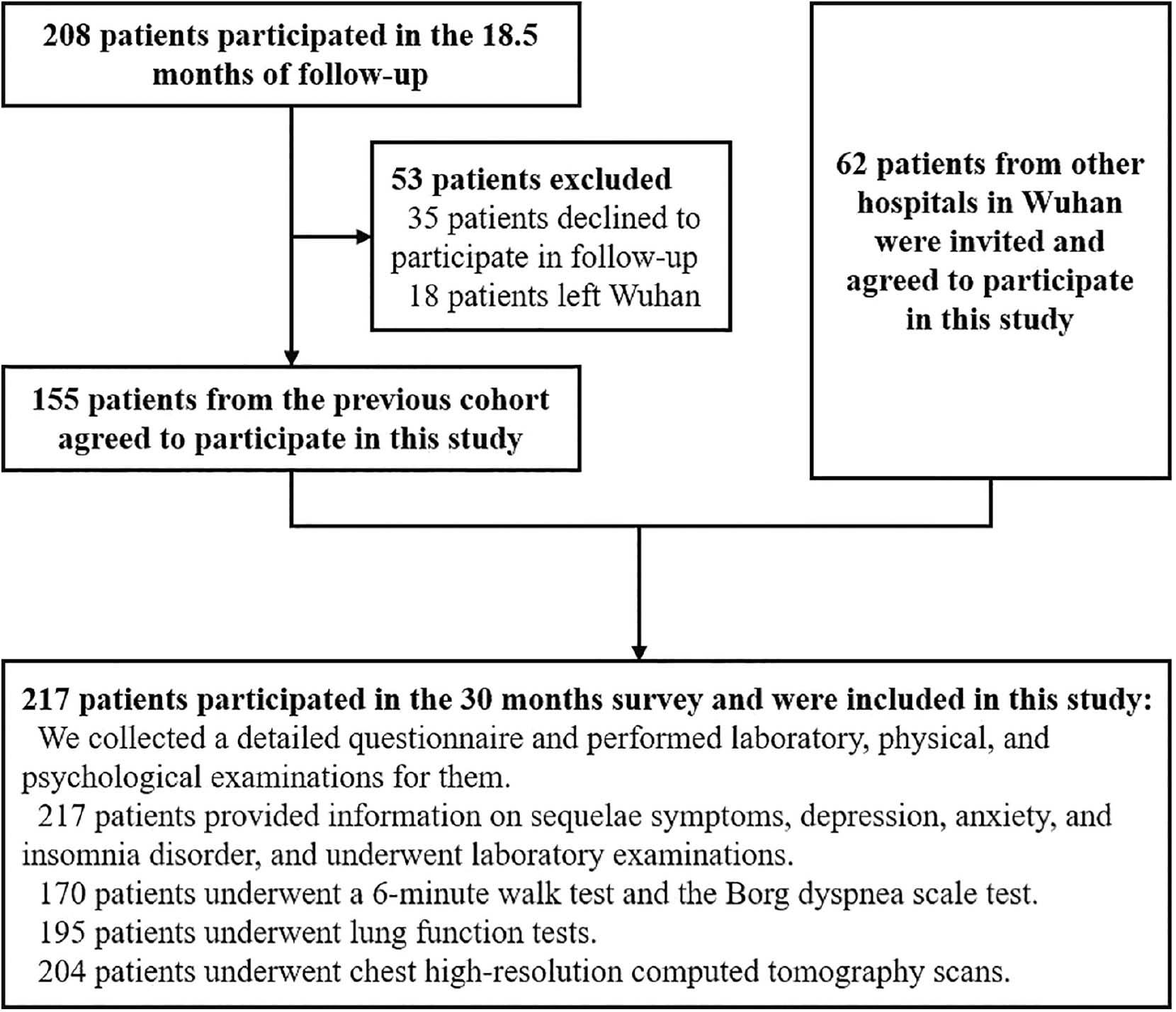
To determine the health consequences in COVID-19 convalescent patients 30 months after infection, we enrolled 217 COVID-19 convalescent patients at Hubei Provincial Hospital of Traditional Chinese Medicine from August–September 2022. Among the 217 convalescent patients, 155 were from an established prospective cohort, the detailed information of which was described in previous studies [17,26]. The other 62 convalescent patients were infected with COVID-19 from January–February 2020 and were admitted to other hospitals in Wuhan. We invited the patients to participate in this survey. None of the 217 convalescent patients were shown to have secondary infections. The convalescent patients completed a detailed questionnaire and the following: laboratory testing; a 6-min walk test (6MWT); the Borg dyspnea scale assessment; lung function testing; and a chest HRCT. A flow chart of the study is presented in Fig 1.
Definitions and data collection
The disease severity of COVID-19 convalescent patients was defined by the Diagnosis and Treatment of Coronavirus Disease-19 (7th trial edition) [27], which was issued on 3 March 2020. This guideline divides the clinical classification of COVID-19 into four categories (mild, moderate, severe, and critical). In the current study we classified mild and moderate cases as mild, and classified severe and critical cases as severe.
Demographic characteristics, co-morbidities, medication history, vaccination status, long COVID symptoms, anxiety, depression, and insomnia were collected from a detailed questionnaire. Routine blood testing, the 6MWT, the Borg dyspnea scale, lung function testing, and chest HRCT were performed by trained professionals.
The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. Participants were divided into 2 groups based on the BMI in the current study (< 24.0 kg/m2 [normal weight group] and ≥ 24.0 kg/m2 [overweight group]). Co-morbidities (hypertension, hyperlipemia, diabetes, cardiovascular disease, chronic obstructive pulmonary disease [COPD], and cancer) and medication history were self-reported. Cardiovascular disease included coronary heart disease and stroke. Cigarette smoking status was categorized as never and ever smokers. Never smokers were those patients who had never smoked, while ever smokers were those patients who smoked in the past but quit smoking and those who are currently smoking. Alcohol intake was categorized into never and ever drinkers. Never drinkers were those who had never consumed alcohol, while ever drinkers were those who used to consume alcohol but quit consuming alcohol and those who are currently consuming alcohol. Exercise was defined as those who regularly participated in at least one type of sport included in the questionnaire (walking, jogging, bicycling, ball games, dancing, Tai Chi, and swimming). Long COVID was defined as one or more symptoms persisting or remitting for > 12 weeks since an acute COVID-19 diagnosis without an alternative explanation [11]. Long COVID included cough, fatigue, a smell or taste disorder, decreased appetite, diarrhea or vomiting, dizziness or headaches, chest pain, and sore throat [11,28].
The 6MWT, Borg dyspnea scale, lung function testing, chest HRCT, anxiety status, and depression status were performed and/or assessed according to a previous study [17]. Briefly, the 6MWT was performed and the distance and predicted percentage of distances were obtained. The Borg dyspnea scale was recorded after the 6MWT [29]. Participants underwent lung function testing before and after the 6MWT. The forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1):FVC ratio, and the forced expiratory flow between 25% and 75% of the vital capacity (FEF25-75%) were collected and these indicators after completion of the 6MWT are shown in the form of a predicted percentage of normal values [30]. The ground-glass opacity (GGO) and reticular pattern (RP) scores were used to assess chest CT scans. GGO and RP scores ranged from 0–24 and 0–26 points, respectively [17]. The definitions of GGO and RP scores are described in detail (S1 and S2 Tables). Abnormal chest CT GGO and RP scores are shown in S1 Fig. Anxiety and depression status were measured and convalescent patients with scores ≥ 5 were considered to have mild or higher levels of anxiety [31] and depression [32].
The insomnia status of COVID-19 convalescent patients was measured according to the Insomnia Severity Index (ISI) scale [33]. The scales are composed of seven items. Each item in ISI is rated on a 0-4 scale, with the total scores ranging from 0-28. Convalescent patients with scores ≥ 8 were considered to have mild or higher levels of insomnia [33].
The five-dimension five-level questionnaire was performed to assess health-related quality of life according to the EQ-5D-5L [34,35] standard and the China EQ-5D-5L value set [36]. The five dimensions included mobility problems, self-care problems, usual activity problems, pain or discomfort, and anxiety or depression. The scoring systems of each problem were as follows: 1, no problems; 2, slight problems; 3, moderate problems; 4, severe problems; and 5, unable to assess or extremely severe problems. The EQ-5Dutility index was converted according to the China EQ-5D-5L value set and indicated good or poor health status (range, 0–1) [36]. EQ-VAS indicated a self-rating of overall health on the day of the interview based on the visual analogue scale (range, 0–100) [34]. Higher scores indicated better health status.
Statistical analysis
Basic characteristics, long-term health consequences, and biochemical indicators of COVID-19 convalescent patients are expressed as medians (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. Basic characteristics, long-term health consequences of convalescent patients, and biochemical indicators were divided into two groups according to the severity of COVID-19 (mild and severe cases) and compared using the Mann-Whitney U test, χ2 test, or Fisher’s exact test, as indicated. The basic characteristics and long-term health consequences and biochemical indicators of convalescent patients were also categorized into four groups based on the the number of vaccine doses and compared using the Kruskal-Wallis test, χ2 test, or Fisher’s exact test, as indicated. Multivariable adjusted logistic regression analysis was performed to calculate the odds ratio (OR) and 95% confidence interval (CI) for the association of risk factors with long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted gender, vaccination status, cigarette smoking, alcohol consumption, exercise habits, and overweight status to determine the association(s) between age and long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, vaccination status, cigarette smoking, alcohol consumption, exercise habits, overweight status, severity of COVID-19, and co-morbidities to determine the association(s) between gender and long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, cigarette smoking, alcohol consumption, exercise habits, overweight status, severity of COVID-19, and co-morbidities to determine the association(s) between vaccination status and long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, vaccination status, alcohol consumption, exercise habits, and overweight status to determine the association(s) between cigarette smoking and long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, vaccination status, cigarette smoking, exercise habits, and overweight status to determine the association(s) between alcohol consumption with long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, vaccination status, cigarette smoking, alcohol consumption, and overweight status to determine the association(s) between exercise habits and long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, vaccination status, cigarette smoking, alcohol consumption, and exercise habits to determine the association(s) between being overweight with long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, vaccination status, cigarette smoking, alcohol consumption, exercise habits, overweight status, and co-morbidities to determine the association(s) between COVID-19 severity with long COVID, anxiety, depression, insomnia, and chest CT abnormalities. We adjusted age, gender, vaccination status, cigarette smoking, alcohol consumption, exercise habits, and overweight status to determine the association(s) between co-morbidities with long COVID, anxiety, depression, insomnia, and chest CT abnormalities. Age was calculated based on 10-year increments in the multivariable-adjusted logistic regression analysis. All tests were two-sided and P values < 0.05 were considered statistically significant. Statistical analyses were carried out using R (version 4.2.1).
RESULTS
Characteristics of participants
The basic characteristics of the study participants are shown in Table 1. Of the 217 participants, 116 had mild cases and 101 had severe cases. The median age of the total population was 59.0 years (IQR, 50.0–66.0). There were 109 (50.2%) males and 108 (49.8%) females. The number of participants who were not vaccinated, received a single vaccine dose, two vaccine doses, and three vaccine doses was 28 (12.9%), 24 (11.1%), 57 (26.3%), and 108 (49.8%), respectively. Hypertension was the most frequent co-morbidity (40.1%), followed by hyperlipemia (36.4%), diabetes (13.8%), cardiovascular disease (13.4%), COPD (3.2%), and cancer (1.8%). The number of participants who were prescribed anti-hypertensive medications, hypoglycemic agents, hypolipidemic agents, and anticoagulants was 77 (35.5%), 25 (11.5%), 44 (20.3%), and 26 (12.0%), respectively. Thirty-one (14.3%) and 40 (18.4%) participants were ever-smokers and ever-drinkers. The number of participants who exercised regularly was 153 (70.5%). There were 85 normal weight participants and 132 overweight participants. We observed no significant differences in the basic characteristics between the mild and severe cases, except for co-morbid cancer. The basic characteristics of COVID-19 convalescent patients according to the number of vaccines received are presented in S3 Table. There were no statistically significant differences between the four groups of patients with the exception of exercise habits. Convalescent patients who received two or three vaccine doses exercised more frequently than patients who were not vaccinated (77.2% or 73.1% vs. 46.4%, P < 0.05).
Basic characteristics of COVID-19 convalescent patients 30 months post-infection.
| Variables | Total population | Mild cases | Severe cases | P value |
|---|---|---|---|---|
| Age, years | 59.0 (50.0, 66.0) | 59.5 (52.0, 66.2) | 59.0 (49.0, 66.0) | 0.270 |
| Female, n (%) | 108 (49.8) | 59 (50.9) | 49 (48.5) | 0.835 |
| Vaccination, n (%) | 0.146 | |||
| Not vaccinated | 28 (12.9) | 10 (8.6) | 18 (17.8) | |
| Single dose | 24 (11.1) | 11 (9.5) | 13 (12.9) | |
| Two doses | 57 (26.3) | 34 (29.3) | 23 (22.8) | |
| Three doses | 108 (49.8) | 61 (52.6) | 47 (46.5) | |
| Co-morbidities, n (%) | ||||
| Hypertension | 87 (40.1) | 52 (44.8) | 35 (34.7) | 0.166 |
| Hyperlipemia | 79 (36.4) | 45 (38.8) | 34 (33.7) | 0.521 |
| Diabetes | 30 (13.8) | 15 (12.9) | 15 (14.9) | 0.832 |
| Cardiovascular disease | 29 (13.4) | 11 (9.5) | 18 (17.8) | 0.109 |
| COPD | 7 (3.2) | 2 (1.7) | 5 (5.0) | 0.255 |
| Cancer | 4 (1.8) | 0 (0.0) | 4 (4.0) | 0.045 |
| Medication administration history, n (%) | ||||
| Anti-hypertensive medication | 77 (35.5) | 45 (38.8) | 32 (31.7) | 0.342 |
| Hypoglycemic agent | 25 (11.5) | 13 (11.2) | 12 (11.9) | 1.000 |
| Hypolipidemic agent | 44 (20.3) | 27 (23.3) | 17 (16.8) | 0.313 |
| Anticoagulant | 26 (12.0) | 12 (10.3) | 14 (13.9) | 0.558 |
| Cigarette smoking, n (%) | 0.677 | |||
| Never-smoker | 186 (85.7) | 101 (87.1) | 85 (84.2) | |
| Ever-smoker | 31 (14.3) | 15 (12.9) | 16 (15.8) | |
| Alcohol consumption, n (%) | 0.173 | |||
| Never-drinker | 177 (81.6) | 99 (85.3) | 78 (77.2) | |
| Ever-drinker | 40 (18.4) | 17 (14.7) | 23 (22.8) | |
| Exercise, n (%) | 153 (70.5) | 87 (75.0) | 66 (65.3) | 0.160 |
| Overweight, n (%) | 132 (60.8) | 72 (62.1) | 60 (59.4) | 0.794 |
Continuous variables are expressed as the median (IQR) and compared with the Mann-Whitney U test. Categorical variables are expressed as a frequency (percentage) and compared by the χ2 test or Fisher’s exact test, as indicated. Abbreviations: COPD, chronic obstructive pulmonary disease. There were 217, 116, and 101 participants in the total population, mild case, and severe case groups, respectively.
Bold P values represent statistically significant results between mild and severe case groups.
Long-term health consequences among COVID-19 convalescent patients
Table 2 demonstrates the long-term health consequences among COVID-19 convalescent patients with respect to long COVID, the 6MWT, the Borg dyspnea scale, lung function testing, chest CT findings, anxiety, depression, insomnia, health-related quality of life, and working status. Of the participants, 62.2% reported that they had at least 1 symptom of long COVID 30 months post-infection. Chest pain was the most reported long COVID symptom (35.0%), followed by fatigue (32.7%) and dizziness or headaches (24.4%). The median (IQR) of distance and predicted percentage of distance were 556.0 meters (IQR, 513.7–602.8 meters) and 98.5% (IQR, 90.0%–105.0%) in the 6MWT, respectively. There were 11 (6.5%) participants with Borg dyspnea scale scores ≥ 1 and who reported dyspnea. The median lung function parameters were 80.5% (IQR, 70.6%–94.2%) for FEV1%, 75.1% (IQR, 64.6%–87.3%) for FVC%, 111.5% (IQR, 102.2%–119.8%) for FEV1/FVC%, and 87.0% (IQR, 67.4%–114.2%) for FEF25-75%. The median CT GGO and RP scores were 1.0 (IQR, 0.0–4.0) and 6.0 (IQR, 2.0–11.0), respectively. The frequency of CT GGO and RP abnormalities were 45.6% and 46.6%, respectively. The median anxiety, depression, and insomnia disorder scores were 1.0 (IQR, 0.0–3.0), 2.0 (IQR, 0.0–4.0), and 4.0 (IQR, 1.0–8.0), respectively. The frequency of anxiety, depression, and insomnia scores ≥5, 5, and 8 were 16.6%, 23.0%, and 32.3%, respectively. The most reported health-related quality of life problem was pain or discomfort (33.2%), followed by anxiety or depression (26.7%). Of the participants, 6.9% and 0.9% reported mobility and visual acuity problems, respectively. None of the convalescent patients reported self-care problems. The median EQ-5Dutility index and EQ-VAS scores were 1.0 (IQR, 0.9–1.0) and 80.0 (IQR, 76.8–80.0), respectively, which indicated that participants in our study had a good quality of life in 30 months post-infection. No significant difference was observed between the mild and severe cases, with the exception of depression disorder and EQ-VAS score. The severe cases had a higher frequency of mild or higher levels of depression compared to the mild cases (29.7% vs. 23%; P = 0.044), and lower median EQ-VAS scores (76.8 vs. 80.0; P = 0.034).
Long-term health consequences of the COVID-19 convalescent patients 30 months post-infection.
| Variables | Total population | Mild cases | Severe cases | P value |
|---|---|---|---|---|
| Long COVID, n (%) | ||||
| Long COVID | 135 (62.2) | 71 (61.2) | 64 (63.4) | 0.852 |
| Cough | 44 (20.3) | 25 (21.6) | 19 (18.8) | 0.740 |
| Fatigue | 71 (32.7) | 37 (31.9) | 34 (33.7) | 0.895 |
| Smell or taste disorder | 24 (11.1) | 16 (13.8) | 8 (7.9) | 0.247 |
| Decreased appetite | 20 (9.2) | 11 (9.5) | 9 (8.9) | 1.000 |
| Diarrhea or vomiting | 11 (5.1) | 6 (5.2) | 5 (5.0) | 1.000 |
| Dizziness or headaches | 53 (24.4) | 28 (24.1) | 25 (24.8) | 1.000 |
| Chest pain | 76 (35.0) | 37 (31.9) | 39 (38.6) | 0.372 |
| Sore throat | 21 (9.7) | 14 (12.1) | 7 (6.9) | 0.295 |
| 6-minute walk test | ||||
| Distance, m | 556.0 (513.7, 602.8) | 556.0 (514.0, 598.0) | 554.5 (511.6, 612.0) | 0.636 |
| Predicted distance % | 98.5 (90.0, 105.0) | 99.0 (90.0, 106.0) | 97.0 (89.0, 103.0) | 0.132 |
| Borg dyspnea scale ≥1, n (%) | 11 (6.5) | 6 (7.1) | 5 (5.9) | 1.000 |
| Lung function | ||||
| FEV1% | 80.5 (70.6, 94.2) | 79.3 (69.1, 92.0) | 81.0 (70.7, 96.0) | 0.329 |
| FVC% | 75.1 (64.6, 87.3) | 75.3 (64.8, 85.5) | 75.1 (64.0, 92.1) | 0.634 |
| FEV1/FVC% | 111.5 (102.2, 119.8) | 108.9 (100.5,118.4) | 113.1 (103.8, 121.0) | 0.114 |
| FEF25-75% | 87.0 (67.4, 114.2) | 83.3 (63.0, 108.8) | 91.8 (69.0, 115.3) | 0.083 |
| Chest CT | ||||
| CT abnormal of GGO, n (%) | 93 (45.6) | 45 (40.9) | 48 (51.1) | 0.190 |
| CT GGO scores | 1.0 (0.0, 4.0) | 1.0 (0.0, 3.0) | 2.0 (0.0, 5.0) | 0.192 |
| CT abnormal of RP, n (%) | 95 (46.6) | 49 (44.5) | 46 (48.9) | 0.627 |
| CT RP scores | 6.0 (2.0, 11.0) | 5.0 (2.0, 10.0) | 6.0 (1.0, 13.0) | 0.394 |
| Anxiety | ||||
| Anxiety disorder, score | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 1.0 (0.0, 3.0) | 0.164 |
| Anxiety score ≥ 5, n (%) | 36 (16.6) | 17 (14.7) | 19 (18.8) | 0.523 |
| Depression | ||||
| Depression disorder, score | 2.0 (0.0, 4.0) | 2.0 (0.8, 3.2) | 2.0 (0.0, 5.0) | 0.760 |
| Depression score ≥ 5, n (%) | 50 (23.0) | 20 (17.2) | 30 (29.7) | 0.044 |
| Insomnia | ||||
| Insomnia disorder, score | 4.0 (1.0, 8.0) | 4.0 (1.0, 8.0) | 4.0 (1.0, 9.0) | 0.861 |
| Insomnia score ≥ 8, n (%) | 70 (32.3) | 38 (32.8) | 32 (31.7) | 0.981 |
| Health-related quality of life | ||||
| Mobility problem | 15 (6.9) | 9 (7.8) | 6 (5.9) | 0.796 |
| Self-care problem | 0 (0) | 1 (0) | 0 (0) | |
| Usual activity problem | 2 (0.9) | 1 (0.9) | 1 (1.0) | 1.000 |
| Pain or discomfort | 72 (33.2) | 33 (28.4) | 39 (38.6) | 0.149 |
| Anxiety or depression | 58 (26.7) | 22 (19.0) | 36 (35.6) | 0.009 |
| EQ-5Dutility index | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) | 0.065 |
| EQ-VAS score | 80.0 (76.8, 80.0) | 80.0 (76.8, 80.0) | 76.8 (73.6, 80.0) | 0.034 |
| Working status | 0.548 | |||
| Unemployment | 7 (3.2) | 5 (4.3) | 2 (2.0) | |
| Work delayed | 3 (1.4) | 1 (0.9) | 2 (2.0) | |
| Decreased income | 17 (7.8) | 8 (6.9) | 9 (8.9) | |
| Busier than before | 1 (0.5) | 0 (0.0) | 1 (1.0) | |
| Made no difference | 111 (51.2) | 56 (48.3) | 55 (54.5) | |
| Retired | 78 (35.9) | 46 (39.7) | 32 (31.7) |
Continuous variables are expressed as the median (IQR) and compared with the Mann-Whitney U test. Categorical variables are expressed as a frequency (percentage) and compared by the χ2 test or Fisher’s exact test, as indicated. Anxiety, depression, and insomnia scores ≥ 5, 5, and 8 were considered to have mild or higher levels of anxiety, depression, and insomnia, respectively. There were 217, 116, and 101 participants in the total population, mild case, and severe case groups, respectively.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25-75%, forced expiratory flow between 25% and 75% of the vital capacity; CT, computed tomography; GGO, ground-glass opacity; RP, reticular pattern
Bold P values represent statistically significant differences.
The long-term health consequences among COVID-19 convalescent patients according to the number of vaccine doses are presented in Table 3. We detected a statistically significant difference in fatigue, anxiety, depression, and insomnia disorder scores, the EQ-5Dutility index, and the EQ-VAS score among the four groups (all P < 0.05). Convalescent patients who received two or three vaccine doses (29.8% or 23.1%, respectively) had significantly less fatigue than patients who were not vaccinated or received a single vaccine dose (50% or 62.5%, respectively). The median anxiety scores were 2.0 (IQR, 1.0–5.0) for the not vaccinated group, which were significantly higher than the median anxiety scores for the convalescent patients who received two or three vaccine doses (median score = 0.0 or 1.0). Similarly, the median score of depression was 4.0 (IQR, 1.8–6.0), which was significantly greater than 1.0 (IQR, 0.0–3.0) in the three-dose group. Convalescent patients who received three vaccine doses had lower median insomnia disorder scores (2.0; IQR, 1.0–7.2) than patients who received two vaccine doses (6.0; IQR 4.0–10.0). Convalescent patients who received three vaccine doses had higher EQ-5Dutility index and EQ-VAS scores than patients who were not vaccinated, with median scores of 1.0 and 80.0, respectively, in the three-dose groups, and 0.9 and 76.8, respectively, in the not vaccinated group.
Long-term health consequences of the COVID-19 convalescent patients according to the number of vaccine doses 30 months post-infection.
| Variables | Not vaccinated | Single dose | Two doses | Three doses |
|---|---|---|---|---|
| Long COVID, n (%) | ||||
| Long COVID | 22 (78.6) | 20 (83.3) | 37 (64.9) | 56 (51.9) |
| Cough | 9 (32.1) | 8 (33.3) | 6 (10.5) | 21 (19.4) |
| Fatigue | 14 (50.0)* | 15 (62.5)£¶ | 17 (29.8) | 25 (23.1) |
| Smell or taste disorder | 3 (10.7) | 2 (8.3) | 9 (15.8) | 10 (9.3) |
| Decreased appetite | 6 (21.4) | 2 (8.3) | 5 (8.8) | 7 (6.5) |
| Diarrhea or vomiting | 1 (3.6) | 1 (4.2) | 4 (7.0) | 5 (4.6) |
| Dizziness or headaches | 11 (39.3) | 4 (16.7) | 15 (26.3) | 23 (21.3) |
| Chest pain | 8 (28.6) | 13 (54.2) | 23 (40.4) | 32 (29.6) |
| Sore throat | 3 (10.7) | 4 (16.7) | 1 (1.8) | 13 (12.0) |
| 6-minute walk test | ||||
| Distance, m | 578.2 (526.8, 611.1) | 560.8 (484.0, 622.6) | 556.0 (517.2, 591.0) | 542.0 (511.6, 596.0) |
| Predicted distance % | 91.5 (82.5, 101.5) | 92.5 (88.0, 99.2) | 99.0 (94.0, 106.0) | 100.0 (90.0, 106.0) |
| Borg dyspnea scale ≥ 1, n (%) | 0 (0) | 0 (0) | 3 (7.3) | 8 (8.6) |
| Lung function | ||||
| FEV1% | 78.0 (65.1, 92.7) | 85.2 (73.5, 101.2) | 83.2 (69.0, 96.4) | 79.2 (71.1, 90.8) |
| FVC% | 70.3 (66.2, 86.1) | 80.0 (71.8, 95.6) | 77.8 (64.8, 90.6) | 74.2 (64.0, 84.8) |
| FEV1/FVC% | 111.4 (103.4, 124.1) | 111.6 (101.6, 118.8) | 111.4 (102.4, 121.5) | 111.6 (102.3, 118.6) |
| FEF25-75% | 83.2 (59.5, 97.4) | 99.6 (74.0, 112.0) | 88.8 (68.3, 118.1) | 86.0 (67.5, 114.3) |
| Chest CT | ||||
| CT abnormal of GGO, n (%) | 13 (54.2) | 8 (40.0) | 23 (42.6) | 49 (46.2) |
| CT GGO score | 2.0 (0.8, 4.2) | 1.0 (0.0, 5.2) | 1.0 (0.0, 3.8) | 1.0 (0.0, 3.8) |
| CT abnormal of RP, n (%) | 13 (54.2) | 11 (55.0) | 30 (55.6) | 41 (38.7) |
| CT RP score | 7.5 (2.8, 12.2) | 7.5 (0.8, 13.0) | 7.0 (2.0, 11.0) | 5.0 (2.0, 10.0) |
| Anxiety | ||||
| Anxiety disorder, score | 2.0 (1.0, 5.0)§* | 1.0 (0.0, 3.0) | 0.0 (0.0, 3.0) | 1.0 (0.0, 2.0) |
| Anxiety score ≥ 5, n (%) | 9 (32.1) | 5 (20.8) | 10 (17.5) | 12 (11.1) |
| Depression | ||||
| Depression disorder, score | 4.0 (1.8, 6.0)* | 3.0 (1.0, 4.2) | 2.0 (1.0, 4.0) | 1.0 (0.0, 3.0) |
| Depression score ≥ 5, n (%) | 11 (39.3) | 6 (25.0) | 13 (22.8) | 20 (18.5) |
| Insomnia | ||||
| Insomnia disorder, score | 4.5 (1.0, 9.0) | 4.5 (0.8, 12.2) | 6.0 (4.0, 10.0)※ | 2.0 (1.0, 7.2) |
| Insomnia score ≥ 8, n (%) | 11 (39.3) | 10 (41.7) | 22 (38.6) | 27 (25.0) |
| Health-related quality of life | ||||
| Mobility problem | 3 (10.7) | 2 (8.3) | 4 (7.0) | 6 (5.6) |
| Self-care problem | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Usual activity problem | 0 (0.0) | 1 (4.2) | 0 (0.0) | 1 (0.9) |
| Pain or discomfort | 13 (46.4) | 8 (33.3) | 22 (38.6) | 29 (26.9) |
| Anxiety or depression | 13 (46.4) | 7 (29.2) | 16 (28.1) | 22 (20.4) |
| EQ-5Dutility index | 0.9 (0.9, 1.0)* | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) |
| EQ-VAS score | 76.8 (73.6, 80.0)* | 80.0 (73.6, 80.0) | 76.8 (76.8, 80.0) | 80.0 (76.8, 80.0) |
| Working status | ||||
| Unemployment | 0 (0.0) | 1 (4.2) | 1 (1.8) | 5 (4.6) |
| Work delayed | 0 (0.0) | 1 (4.2) | 0 (0.0) | 2 (1.9) |
| Decreased income | 4 (14.3) | 5 (20.8) | 4 (7.0) | 4 (3.7) |
| Busier than before | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) |
| Made no difference | 20 (71.4) | 10 (41.7) | 27 (47.4) | 54 (50.0) |
| Retired | 4 (14.3) | 7 (29.2) | 25 (43.9) | 42 (38.9) |
Continuous variables are expressed as the median (IQR) and compared with the Kruskal-Wallis test. Categorical variables are expressed as a frequency (percentage) and compared by the χ2 test or Fisher’s exact test, as indicated. Anxiety, depression, and insomnia scores ≥5, 5, and 8 were considered to have mild or higher levels of anxiety, depression, and insomnia, respectively. §Significant difference between the not vaccinated and two-dose groups (P < 0.05). *Significant difference between the not vaccinated and three-dose groups (P < 0.05). £Significant difference between the single and two-dose groups (P < 0.05). ¶Significant difference between the single and three-dose groups (P < 0.05). ※Significant difference between the two- and three-dose groups (P < 0.05). There were 28, 24, 57, and 108 participants in the not vaccinated, single dose, two dose, and three dose groups, respectively.
Abbreviations: FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25-75%, forced expiratory flow between 25% and 75% of vital capacity; CT, computed tomography; GGO, ground-glass opacity; RP, reticular pattern.
Potential risk factors for health consequences among COVID convalescent patients
Fig 2 illustrates the potential risk factors for long COVID, anxiety, depression, insomnia, and chest CT abnormalities among the convalescent patients. After multivariable adjustment, the increase 10-year increment of age was independently associated with higher risks of long COVID (OR = 1.52, 95% CI = 1.16–2.02) and chest CT abnormalities (OR = 1.75, 95% CI = 1.33–2.36) among participants 31–81 years of age. Female gender was significantly associated with anxiety and depression disorders. Females had a 3.20-fold higher risk of anxiety (OR = 3.20, 95% CI = 1.24–9.16) and 2.49-fold higher risk of depression compared to males (OR = 2.49, 95% CI = 1.11–5.92). Convalescent patients who exercised regularly had a lower risk of anxiety (OR = 0.41, 95% CI = 0.18–0.93) compared to patients who did not exercise. Vaccination was associated with a lower risk of long COVID, anxiety, and depression. Convalescent patients who received three vaccine doses had an 82%, 78%, and 67% lower risk of long COVID (OR = 0.18, 95% CI = 0.06–0.50), anxiety (OR = 0.22, 95% CI = 0.07–0.71), and depression (OR = 0.33, 95% CI = 0.12–0.92) than patients who were not vaccinated.

Association of risk factors with long COVID, anxiety, depression, insomnia, and abnormality of chest CT at 30 months after infection. (A) Long COVID. (B) Anxiety. (C) Depression. (D) Insomnia. (E) Abnormality of chest CT. ORs were derived from Logistic regression models. For the association of age with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted gender, vaccination, smoking, alcohol, exercise, and overweight. For the association of gender with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, vaccination, smoking, alcohol, exercise, overweight, severity of COVID-19, and comorbidities. For the association of vaccination with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, smoking, alcohol, exercise, overweight, severity of COVID-19, and comorbidities. For the association of smoking with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, vaccination, alcohol, exercise, and overweight. For the association of alcohol with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, vaccination, smoking, exercise, and overweight. For the association of exercise with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, vaccination, smoking, alcohol, and overweight. For the association of being overweight with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, vaccination, smoking, alcohol, and exercise. For the association of the severity of COVID-19 with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, vaccination, smoking, alcohol, exercise, overweight, and comorbidities. For the association of comorbidities with long COVID, anxiety, depression, insomnia, and abnormality of chest CT, we adjusted age, gender, vaccination, smoking, alcohol, exercise, and overweight. Abbreviations: OR, odds ratio; COPD, chronic obstructive pulmonary disease; ref, reference; CT, computed tomography.
Biochemical indicators of participants
The biochemical indicators among COVID-19 convalescent patients according to disease severity and number of vaccine doses are summarized in S4 and S5 Tables. All indicators were in the normal reference range. Severe cases had higher values of albumin and globulin ratios than mild cases, with a median value of 1.8 in severe cases and 1.7 in mild cases (P = 0.015). Participants who received three vaccine doses had higher values of total cholesterol and lower values of creatinine compared to patients who were not vaccinated, with median values of 4.7 and 61.5, respectively, in the three-dose group, and 4.2 and 71.0, respectively, in the not vaccinated group (all P < 0.05).
DISCUSSION
This is the longest study to investigate the health consequences of COVID-19 convalescent patients for > 30 months post-infection. The majority of convalescent patients were in good overall health and returned to work, while long COVID, anxiety, depression, insomnia, and chest CT abnormalities were sustained for up to 30 months. We further explored the risk factors for the health consequences in the current study. Older age, female gender, physically inactive, and no vaccination were associated with adverse consequences in the convalescent patients. Notably, we observed that receiving three vaccine doses after infection was associated with a lower risk of developing long COVID, and anxiety and depression disorders. Policymakers should be aware of the protective role of post-infection vaccination, which may contribute to the early prevention and management of long-term COVID-19 health consequences and relieve the heavy burden of long COVID.
Long COVID persisted in > 60% of the convalescent patients, with chest pain, fatigue, and dizziness or headaches the most reported symptoms. Similarly, it was reported in a review that these were the predominant symptoms of long COVID [37]. At 30 months post-infection, COVID-19 symptoms remained high in our convalescent patients. Similarly, in a 2-year follow-up study that included 1119 COVID-19 survivors, the frequency of long COVID was 68% at 6 months, 49% at 1 year, and 55% at 2 years [7]. There were 16.6%, 23%, and 32.3% of the total population with anxiety, depression, and insomnia disorders 30 months post-infection. Similar mental health impairments and sleep difficulties were also observed in a 2-year follow-up study of COVID-19 [7]. In our 30-month survey, the proportion of long COVID and impaired mental health remained very high. The possible interpretations of these findings include sustained immune dysregulation, microbiota disruption, and dysfunctional signaling in the brainstems of COVID-19 convalescent patients [37]. A previous study suggested that immune dysregulation driven by SARS-CoV-2 during acute COVID-19 not only allows previously harbored pathogens to reactivate, infect new body sites, and drive new symptoms, but also promotes the collective imbalance of the microbial and viral ecosystems within the body, which could disrupt the protective functions of the immune system in the long-term [38]. SARS-CoV-2 may invade the brainstem, which has nuclei controlling symptoms, such as sleep problems, nausea, pain, sickness, and dysautonomia. Such persistent brainstem inflammation or infection may drive long COVID symptoms [38]. In addition, the COVID-19 restrictions were fully lifted at the beginning of December 2022 in China and the 30-month survey was conducted from August–September 2022, therefore these participants may feel more anxious and insecure. The patients were more likely to report long COVID symptoms and mental disorders than before due to the lifting of pandemic restrictions and the fear of being reinfected.
Additionally, the residual lung lesions were sustained with a median FVC% < 80% of the predicted value and CT abnormalities were present in nearly one-half of the convalescent patients. Atelectasis, ongoing alveolitis, and parenchymal fibrosis occurred as a result of post-pulmonary inflammation as COVID-19 progressed [39]. A follow-up study that included 155 two-year COVID-19 survivors also suggested that radiographic abnormalities were still present in 50.7% of patients; fibrosis-like lesions and residual GGOs were most common [18]. Given COVID-19 involves multi-organ damage (e.g., heart, lungs, and the neurologic system) [40], the long-term sequelae symptoms could result in severe complications [37,41]. Some symptoms of long COVID may last for years [42] or even lifelong [43]. More longitudinal studies with large sample sizes are urgently needed to unravel the underlying mechanisms.
In the current study age was shown to be a risk factor for long COVID and chest CT abnormalities. A study conducted in England showed that the risk of long-term symptoms post-SARS-CoV-2 infection increased with age per decade of life [44]. With an increase in age, immunity weakens and individuals become more vulnerable to viral infections [45], followed by more sequelae symptoms. Prior studies have suggested that increasing age, especially ≥ 50 years, is a risk factor for residual CT abnormalities, which is in agreement with our study findings [46,47]. Female gender was associated with a higher risk of anxiety and depression disorders in this study. A longitudinal cohort study including 1276 one-year COVID-19 survivors also showed that females had higher risks of anxiety or depression than males (OR = 2.00, 95% CI = 1.48–2.69) [6]. Females are more vulnerable to mental health disorders due to genetics and steroid hormones [48], physiologic states, and perceived stress levels [49]. Moreover, convalescent patients who exercised regularly had less anxiety than patients who did not exercise regularly. Existing evidence suggests that physical activity is directly associated with lower anxiety scores (β = -0.03, P = 0.04) caused by COVID-19 [50], while physical inactivity is associated with an increased risk of anxiety (OR = 2.18, 95% CI = 1.72–2.78) [51]. Physical activity may improve anxiety symptoms through the regulation of inflammatory systems [52], upregulation of brain-derived neurotrophic factor [53], and neurogenesis and angiogenesis improvement [54,55]. Because exercise can be helpful to alleviate anxiety, it should be encouraged and recommended reasonably in COVID-19 convalescent patients.
It has been acknowledged that patients who were vaccinated before SARS-CoV-2 infection had lower risks of sequelae symptoms [21], while only a few studies determined if receiving a vaccine after infection reduced the risk of long COVID [56,57]. In the present study we observed that three vaccine doses after infection was associated with a lower risk of long COVID, anxiety, and depression; however, the association between vaccination and anxiety should be interpretated cautiously. The actual reasons (e.g., concern about COVID-19 and concern about vaccine side effects) for anxiety among convalescent patients is unknown. A survey including 1596 participants reported that vaccination (one or two doses) after infection was associated with a decreased risk of post-SARS-CoV-2 symptoms (e.g., fatigue, difficulty concentrating or memory loss, shortness of breath, and headaches) after a median > 250 days of infection (OR = 0.72, 95% CI = 0.56–0.92) [57]. A community-based cohort study in Britain indicated that adults who received one or two vaccine doses post-infection had a lower risk (OR = 0.87, 95% CI = 0.81–0.93 and OR = 0.91, 95% CI = 0.86–0.96, respectively) of long COVID symptoms over a median of 267 days after infection [56]. The hypothesized mechanisms of long COVID may include viral remnants. These viral remnants, such as fragments of virus and protein molecules, could disrupt the body for months [58]. In addition, the immune system was dysregulated and attacked the rest of the body, which also may cause long COVID [58]. According to previous research, the vaccine improved long COVID symptoms by eliminating the viral remnants and accelerating the clearance of SARS-CoV-2 [58]. Furthermore, the vaccine also helped rebalance the immune system [58] and reduced the exaggerated inflammation [59]. Our study findings were consistent with the findings of previous studies and further extended the duration to 30 months post-infection.
It should be noted that one or two vaccine doses in other studies afforded protection against long COVID compared to three vaccine doses in the current study. Inactivated virus, mRNA, adenovirus vector-based, and adjuvanted protein vaccines were widely used during the epidemic [60]. Different types of vaccine technologies have different mechanisms of action [61] and vaccine durability [60]. Specifically, inactivated and mRNA vaccines produce different levels of cellular and humoral immunity [62]. All of the vaccinated participants in the current study received an inactivated vaccine. Therefore, the reasons for the differences in immunity may not be due to the different vaccine technologies. The research timing is an important factor influencing the protective effect of the vaccine because the efficacy of the COVID-19 vaccine decreases over time [63]. A study from China that included 61 participants who received the inactivated CoronaVac vaccine showed that the ability to protect against SARS-CoV-2 infection was poor 160 days after vaccination [64]. A phase 2 clinical trial involving the inactivated CoronaVac vaccine also showed that participants who received two CoronaVac doses had a lower neutralizing antibody response after 6 months [65]. Longitudinal studies and clinical trials are required to confirm our findings and elucidate the biological mechanisms underlying the protective impact of vaccination in long COVID development.
The median values of albumin and globulin ratios in the mild and severe case groups were in the normal range. Similarly, the median total cholesterol and creatinine levels were in the normal range for the groups that were not vaccinated and received three doses. Although there were statistical differences in albumin and globulin ratio between the mild and severe case groups, and in the total cholesterol and creatine levels between the groups that were not vaccinated and received three doses, these results must be interpreted with caution. Whether there is a clear biological significance between the groups needs to be elucidated in other studies.
This is the longest study to investigate the health consequences among COVID-19 convalescent patients. We have reported multiple comprehensive health outcomes and the potential risk factors 30 months after infection. In addition, we observed the protective role of vaccination after infection in the long-term health consequences of COVID-19 in a real-world scenario.
This study has several limitations. First, we did not measure the transfer factor for carbon monoxide (TLCO) in the lung function test. A thorough lung function test should be performed to provide more information. Second, our study was based on a cross-sectional design, thus more investigations with large and longitudinal cohorts are warranted to confirm our findings. Third, the SARS-CoV-2 virus mutates over time, and one should be cautious when generalizing our findings to other variants [66–69]. Our participants were infected with the wild-type strain, and the sequelae symptoms of emerging variants (e.g., alpha, beta, gamma, delta, and omicron) are uncertain and warrant further investigation [70]. Of the convalescent patients, 62.2% (wild-type strain) had symptoms of long COVID 30 months post-infection, while a recent retrospective cohort study conducted in India reported that only 8.2% of the omicron cases had long COVID after a median of 73 days post-infection [71]. Even though various variants lead to different types and severity of long COVID symptoms, our study provides important evidence for the early prevention and targeted treatment of COVID-19 health consequences. Fourth, the policy of epidemic prevention and control have experienced dynamic changes from 2020–2022. The psychological condition could differ in patients who were infected earlier and later. The lengths of recovery time after infection were quite different for the patients, which also influenced the current psychological conditions of COVID-19 convalescent patients [19]. The aim of the present study was to determine the long-term consequences of COVID-19, and we only included patients who were infected in the early stage of the epidemic. In a future corollary study we may compare mental health disorders in patients who were infected in different stages of the epidemic and with different lengths of recovery time.
In conclusion, our findings indicated that most convalescent patients were in good overall health and returned to work, while some symptoms of long COVID (anxiety, depression, insomnia, and chest CT abnormalities) were sustained for up to 30 months. Convalescent patients who were older, female, physically inactive, and not vaccinated were more susceptible to the adverse consequences of COVID-19. It is also necessary to highlight the importance of vaccination after infection given its protective role in the development of long-term consequences of COVID-19. Our findings provide novel evidence for policymakers on the multiple health consequences and risk factors 30 months post-infection. Implementing targeted prevention and treatment measures to combat the long-term COVID-19 consequences is a pressing priority.