Abbreviations: TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TNFα, tumor necrosis factor α; IL-6, interleukin-6; IL-1β, interleukin-1β; MCP-1, monocyte chemotactic protein 1; SOD, superoxide dismutase; GSH-PX, glutathione peroxidase; MMP-9, matrix metalloproteinase-9; VCAM-1, vascular cell adhesion molecule-1; AP-1, nuclear transcription factor activating protein-1; ROS, reactive oxygen species; NOX4, NADPH oxidase 4; GST, glutathione transferase; eNOS, endothelial nitric oxide synthase; NLRP3, Nod-like receptor protein 3.
Introduction
Atherosclerosis (AS), the major cause of atherosclerotic cardiovascular disease (ASCVD), is a chronic inflammatory disease of the arteries driven by dyslipidemia and characterized by endothelial dysfunction, vascular wall inflammatory infiltration, lipid accumulation, and plaque formation [1]. To date, ASCVD remains the main cause of morbidity and mortality worldwide, thus posing a major economic burden on individuals and families [2]. Therefore, new ASCVD treatments are urgently needed.
Sodium-dependent glucose cotransporter (SGLT) has two main subtypes: SGLT1 and SGLT2. SGLT1 is expressed primarily in the small intestine, liver, lung, kidney, and heart, whereas SGLT2 is expressed primarily in the kidneys. SGLT2 inhibitors are a new type of anti-hyperglycemic drug that elicits hypoglycemic effects through inhibiting glucose reabsorption by the proximal curved tubules and increasing urinary glucose excretion [3]. Several recently completed large randomized clinical trials have shown that SGLT2 inhibitors also have cardiovascular protective effects [4–6], although the underlying mechanisms are not fully understood. Treatment with an SGLT2 inhibitor has been associated with diminished risk of major adverse cardiovascular events in patients with type 2 diabetes mellitus (T2DM) and ASCVD [7]. Hence, SGLT2 inhibitors may have roles in the prevention and treatment of AS. Recent studies have shown that SGLT2 inhibitors have anti-AS functions by promoting cholesterol excretion, improving endothelial cell function, and exerting anti-inflammatory effects [8–10]. However, the role of SGLT2 inhibitors against AS and the underlying mechanisms are not fully understood.
Macrophages play important roles in AS occurrence and development, and are closely associated with the formation of atherosclerotic plaques [11]. Attracted by adhesion molecules on the surfaces of injured endothelial cells, monocytes in the blood migrate to the intima of injured arteries, differentiate into macrophages, produce pro-inflammatory factors, phagocytose low-density lipoprotein cholesterol (LDL-C), and gradually change into foam cells that form the necrotic cores of atherosclerotic plaques [12]. During this process, the expression of MCP-1, VCAM-1, and other cytokines involved in AS development significantly increase [13]. Similarly to cholesterol lowering, inhibiting these inflammatory components can effectively decrease atherosclerotic lesions [14, 15].
One of the earliest events in the development of AS is LDL oxidation; in mice and humans, NADPH oxidase (NOX) has been found to play a key role in directly or indirectly generating reactive oxygen species (ROS). ROS are used as a substrate by other enzymes, thereby producing stronger oxidative stress [16, 17]. Beyond causing oxidative stress, ROS have been extensively reported to be an early inducer of autophagy [18]. Autophagy, along with ROS overproduction, is integral to the development and progression of AS, and may aid in the identification of new therapeutic targets [19].
Apolipoprotein E knockout (ApoE−/−) mice are widely used in the study of AS, because of their susceptibility to spontaneous hypercholesterolemia and atherosclerotic plaque formation induced by high-fat diets [20]. In this study, we constructed an AS model by feeding ApoE−/− mice a Western diet (WD) high in fat and sugar. We observed the therapeutic effects of canagliflozin (Cana), an SGLT2 inhibitor, on AS, and subsequently explored the possible underlying mechanisms in terms of inflammation, oxidative stress, and autophagy. Our findings may provide a theoretical basis for the prevention and treatment of ASCVD with SGLT2 inhibitors.
Materials and Methods
Animals and Treatment
Male ApoE−/− mice (7–8 weeks old) were provided by Beijing Charles River Laboratory Animal Technology Co., Ltd (Beijing, China). All mice were acclimated in a specific-pathogen-free room at a temperature of 22–24 °C and a humidity of approximately 55%. After 1 week of adaptive feeding of a normal diet, all mice were fed a WD (D12079B, 41% fat and 1.5‰ cholesterol), and were randomly divided into a WD group (n = 8) and a WD+Cana intervention group (n = 8). Cana (Janssen-Cilag International NV, Xian, China) was dissolved in 0.5% hydroxypropyl methylcellulose (0.5%, Coolaber, Beijing, China). Mice in the WD+Cana group were intragastrically administered Cana (20 mg/kg) each day, whereas mice in the WD group received the same dose of 0.5% hydroxypropyl methylcellulose. During the experiment, the body mass of the mice was measured weekly, and fasting blood glucose (FBG) was measured with a glucometer every 4 weeks. After 15 weeks, all mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg) and euthanized, and blood samples, and heart and aorta tissues were collected.
Measurement of Serum Indicators
Serum levels of fasting insulin (FINS) and adiponectin were detected with mouse insulin (Taicang, Shanghai, China) and adiponectin (Elabscience, Wuhan, China) ELISA kits, respectively. Serum levels of TG, TC, HDL-C, LDL-C, SOD, and GSH-PX were measured with colorimetric kits (Nanjing Jiancheng, Nanjing, China). Concentrations of inflammatory cytokines in the serum, including TNF-α, IL-6, IL-1β, and MCP-1, were detected with ELISA kits (Lianke, Hangzhou, China).
Oil Red O Staining
Aortic roots in the heart were processed into 10 μm thick frozen sections. For evaluation of AS, the aortas and frozen sections of aortic roots were stained with Oil Red O. Finally, the lipid deposition area and percentage plaque area were quantified with Image-pro plus 6.0 software (Media Cybermetrics, Rockville, MD, USA).
HE and Masson Staining
Aortic roots were embedded in paraffin, then sliced into 4 μm sections. Subsequently, the slices were dewaxed, rehydrated, and stained with either hematoxylin-eosin (HE) or Masson’s stain. The percentage plaque area and relative content of collagen were quantified with Image-pro plus 6.0 software (Media Cybermetrics, Rockville, MD, USA).
ROS Staining
The frozen sections of the aortic root were rewarmed at room temperature and dehydrated. A hydrophobic barrier pen was used to draw circles around the tissues. Next, spontaneous fluorescence quencher (G1221, Servicebio, Wuhan, China) was added, and ROS dye (D7008, Sigma-Aldrich, Shanghai, China) was added within the circles. After 30 minutes of incubation at 37 °C in the dark, we restained the tissues with DAPI dye (G1012, Servicebio, Wuhan, China). The relative ROS levels were quantified in Image-pro plus 6.0 software (Media Cybermetrics, Rockville, MD, USA).
Immunohistochemistry Assays
The methods and steps were the same as described in our previous study [21]. The slides were incubated first with anti-F4/80 (GB113373, 1:800, Affinity, Wuhan, China) overnight, then with anti-rabbit IgG-HRP (GB23303, 1:200, Servicebio, Wuhan, China), after being washed three times with PBS [21]. Next, the sections were stained with a 3,3′-diaminobenzidine kit (ZLI-9017, Zhongshan, Beijing, China) [21]. The relative F4/80 level was quantified in Image-pro plus 6.0 software (Media Cybermetrics, Rockville, MD, USA).
Immunofluorescence Assays
Paraffin-embedded aortic root slides (4 μm) were dewaxed for antigen repair and sealed with serum, and the slices were incubated overnight at 4 °C with anti-LC3 A/B (4108S, 1:200, Cell Signaling Technology, Beverly, MA, USA) and anti-p62 (ab109012, 1:1000, Abcam, Cambridge, UK) successively. After being washed, the slides were incubated with anti-rabbit IgG-HRP (MF094-01, Mei5 Biotechnology, Beijing, China) at room temperature for 50 min. Finally, DAPI dye (G1012, Servicebio, Wuhan, China) was added to the slides for restaining nuclei. The relative fluorescence intensity was quantified in ImageJ software (NIH, Bethesda, MD, USA).
Quantitative Real-Time PCR Assays
The PCR assay was as described in our previous article [21]. Total RNA was extracted with TRIzol Universal Reagent (Tiangen, Beijing, China), and RNase-free DNase I (Tiangen, Beijing, China) was used to remove DNA [21]. After quality assessment, cDNA was synthesized with ReverTra Ace reverse transcriptase (Tiangen, Beijing, China), and real-time PCR was conducted with SuperReal PreMix Plus (Tiangen, Beijing, China) [21]. GAPDH was used as an internal control for quantification of mRNA levels. All samples were tested in technical triplicate. Primer sequences are listed in Table 1. The 2−ΔΔCt method was applied to calculate relative mRNA expression levels.
Sequences of Primers.
| Gene | Primer Sequence (Forward) | Primer Sequence (Reverse) |
|---|---|---|
| F4/80 | GAATCTAGTGGAGGAGGGCC | ATGAGCAGCTGTAGGATCCC |
| MCP-1 | ACCTTTTCCACAACCACCT | GCATCACAGTCCGAGTCA |
| MMP-9 | GGCTGTTCTGGAGATTGG | CTGGAAGATGTCGTGTGAGTT |
| VCAM-1 | TACTGTTTGAGTCTCTCAAGC | TCACCTTCGCGTTTAGTGGG |
| AP-1 | CCTTCTACGACGATGCCCTC | GGTTCAAGGTCATGCTCTGTTT |
| ROS | GTTTCTGGATGGCTCTGCCT | TGCTGAAAAATGGCCTTGCC |
| NOX4 | GGGATTTGCTACTGCCTCCA | GAGTGACTCCAATGCCTCCA |
| Nrf2 | TAGATGACCATGAGTCGCTTGC | GCCAAACTTGCTCCATGTCC |
| GST | CTCAGGCAGCTCATGGACAAT | GTTATCCTCTGGAATGCGGTC |
| eNOS | GGATCAGCAACGCTACCA | TATGCGGCTTGTCACCTC |
| LC3B | ATGGTGCGATCAGTAAGGA | CAGGTTCGTTGTGCCTTT |
| P62 | CTGCCCTCAGCCCTCTA | TGTCTTCTGTGCCTGTGC |
| NLRP3 | TCTGCACCCGGACTGTAAAC | CATTGTTGCCCAGGTTCAGC |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| GAPDH | CGTCCCGTAGACAAAATGGT | TTGATGGCAACAATCTCCAC |
Results
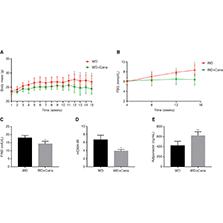
Canagliflozin Alleviates Body Mass and Insulin Resistance in ApoE−/− Mice
The average body mass of mice in the WD+Cana group was consistently lower than that in the WD group. The longer the intervention period, the more significant the mass difference; the difference in the final 4 weeks reached statistical significance (Figure 1A). During the experiment, FBG was monitored in ApoE−/− mice. FBG in the WD group increased gradually, whereas that in the WD+Cana group remained virtually unchanged (Figure 1B). At weeks 12 and 15, FBG was significantly lower in the WD+Cana group than the WD group (P < 0.05). Similarly, serum FINS level and homeostasis assessment of insulin resistance (HOMA-IR, given by glucose (mmol/L) × insulin (mIU/L)/22.5), measured and calculated at the end of week 15, indicated that both indices were significantly lower in the WD+Cana group than the WD group (Figure 1C, D). These results suggested that Cana ameliorated insulin resistance in ApoE−/− mice fed a WD. Furthermore, the serum adiponectin level, which is closely associated with obesity and insulin resistance, was significantly higher in the WD+Cana group than the WD group (P < 0.01, Figure 1E).
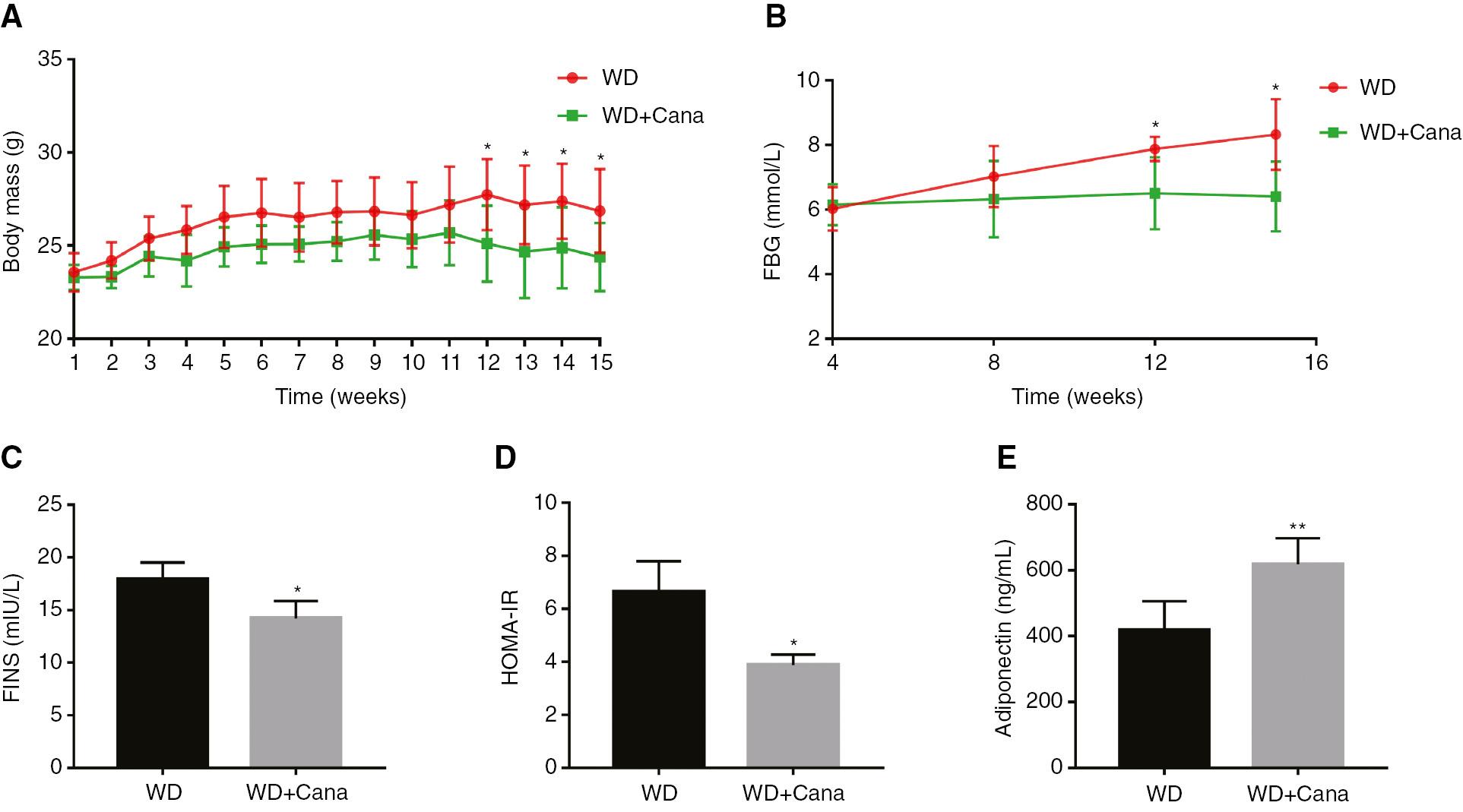
Canagliflozin Decreases Body Mass and Insulin Resistance in ApoE−/− Mice.
(A) Curves of body mass change. n = 8. (B) Curves of FBG, comparing the two groups. n = 4. (C) FINS level. n = 4. (D) HOMA-IR level. n = 4. (E) Adiponectin level. n = 6; *,**P < 0.05, P < 0.01 versus the WD group. FBG, Fasting blood glucose; FINS, Fasting insulin; HOMA-IR, Homeostasis model assessment of insulin resistance.
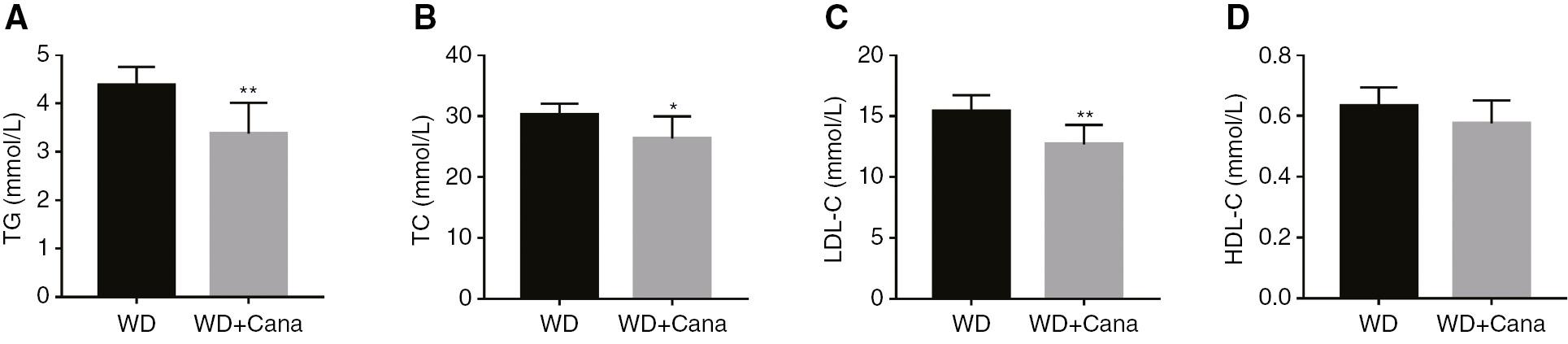
Canagliflozin Ameliorates the Lipid Profile of ApoE−/− Mice
Treatment with 15 weeks of Cana intervention significantly decreased the serum levels of TG, TC, and LDL-C in ApoE−/− mice fed a WD (Figure 2A–C) but had no significant effect on HDL-C (Figure 2D). These lipid composition changes might inhibit the development of AS.
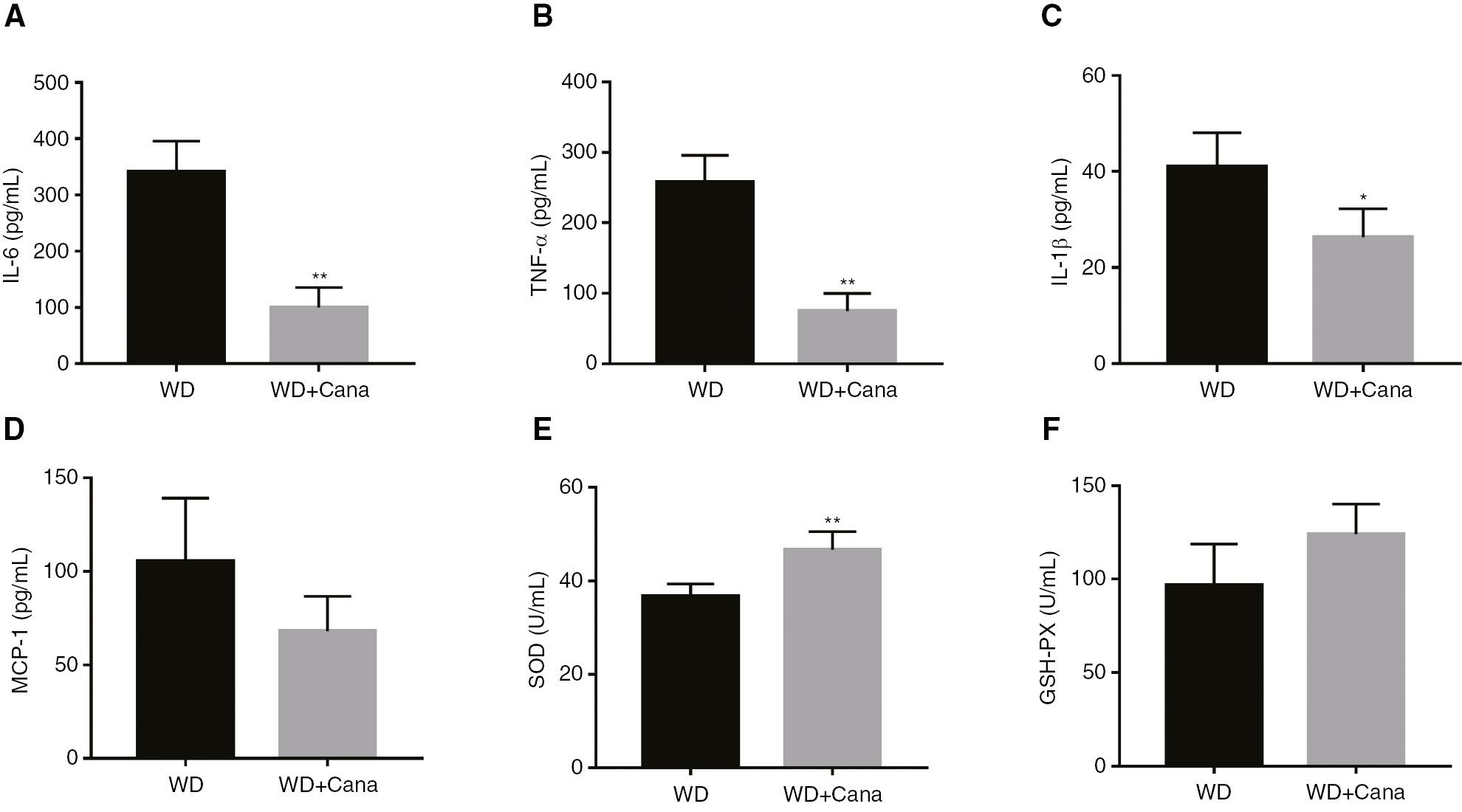
Canagliflozin Decreases Systemic Inflammation and Oxidative Stress in ApoE−/− Mice
Measurements of serum inflammatory cytokines indicated that Cana significantly decreased the serum levels of TNF-α, IL-6, and IL-1β in ApoE−/− mice (Figure 3A–C). In addition, the WD+Cana group exhibited lower MCP-1 levels than the WD group, although the difference did not reach statistical significance (P = 0.063, Figure 3D). Because oxidative stress often indicates inflammation, we further tested the serum levels of the anti-oxidative stress factors SOD and GSH-PX. Both levels were higher in the WD+Cana group than in the WD group (Figure 3E, F).
Canagliflozin Decreases the Degree of Atherosclerotic Damage in ApoE−/− Mice
Pathological analysis indicated that the degree of AS in ApoE−/− mice significantly decreased after 15 weeks of treatment with Cana (Figure 4A). Oil red O staining and HE staining of the aortic root in the WD+Cana group showed a smaller atherosclerotic plaque area than observed in the WD group (Figure 4B, D). A statistically significant difference was observed in the percentage plaque area with respect to the lumen area (Figure 4C, E). Furthermore, Masson staining indicated higher collagen fiber content in the WD+Cana group than the WD group (P = 0.104, Figure 4F, G).
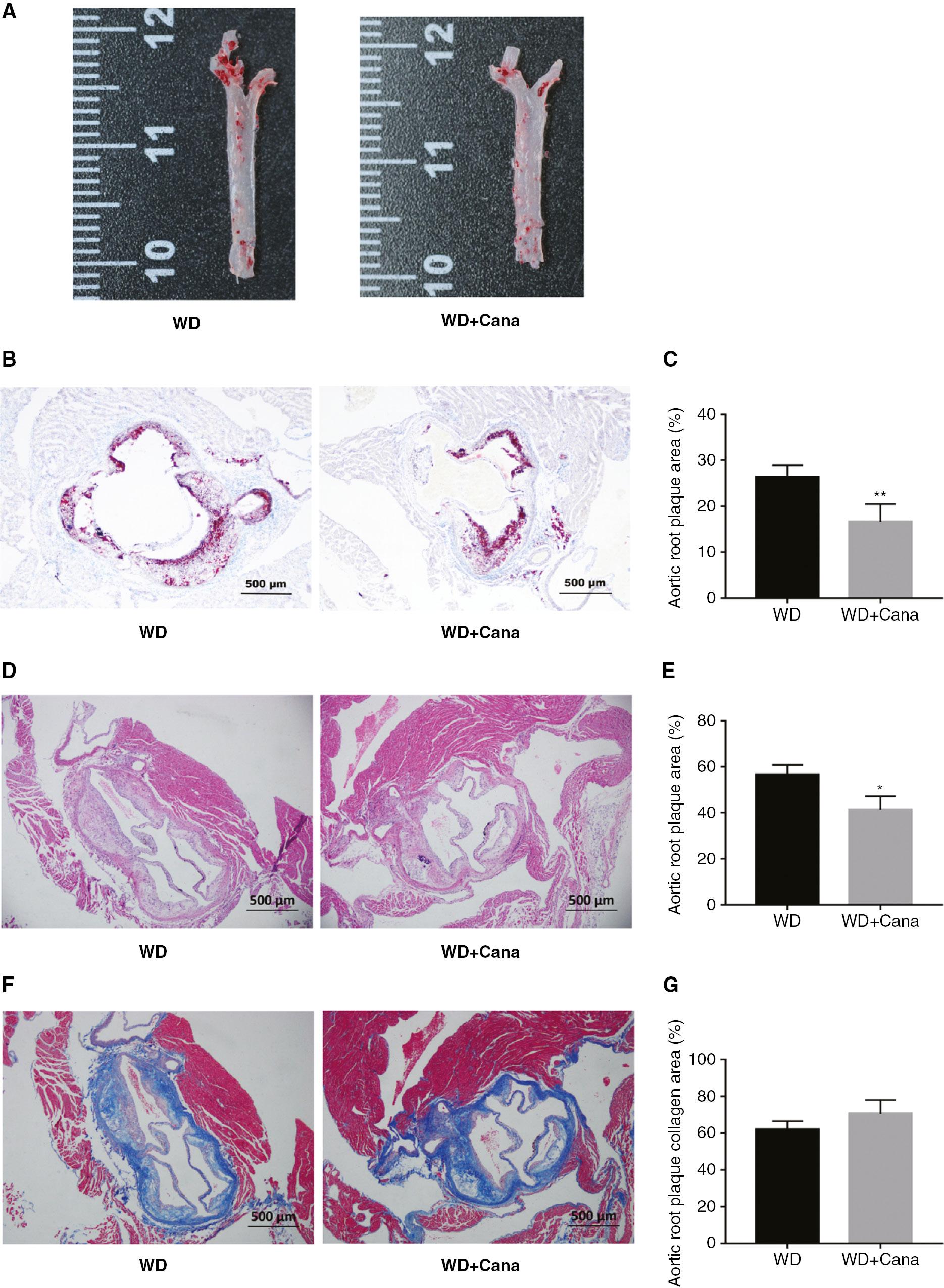
Canagliflozin Decreases the Degree of Atherosclerotic Damage in ApoE−/− Mice.
(A) Representative images of aortas stained with Oil Red O. (B, C) Representative images of aortic roots and the plaque area ratio; tissues were stained with Oil Red O (×40 magnification. n = 4). (D, E) Representative images of aortic roots and the plaque area ratio; tissues were stained with HE (×40 magnification. n = 4). (F, G) Representative images of aortic roots and the collagen area ratio; tissues were stained with Masson’s stain (×40 magnification. n = 4); *,**P < 0.05, P < 0.01 versus the WD group.
Canagliflozin Mitigates Aortic Inflammation in ApoE−/− Mice
AS is a chronic inflammatory disease whose progression is promoted primarily by macrophages. We studied the effect of Cana on inflammatory cell infiltration in aortic root plaques by immunohistochemistry, and found a significantly lower count of F4/80-positive cells in the aortic root in the WD+Cana group than the WD group (P < 0.05, Figure 5A, B). In addition, we further detected the aortic expression of the genes F4/80, MCP-1, MMP-9, VCAM-1, and AP-1, which are closely associated with inflammation. As shown in Figure 5C, after 15 weeks of Cana intervention, the gene expression of F4/80, MCP-1, MMP-9, and AP-1 in the aorta was significantly attenuated with respect to that in the WD group, whereas VCAM-1 expression did not significantly differ between groups (P = 0.053). In conclusion, Cana significantly decreased the infiltration of macrophages in ApoE−/− mice atherosclerotic plaques, diminished the expression of pro-atherosclerotic related cytokines, and enhanced plaque stability.
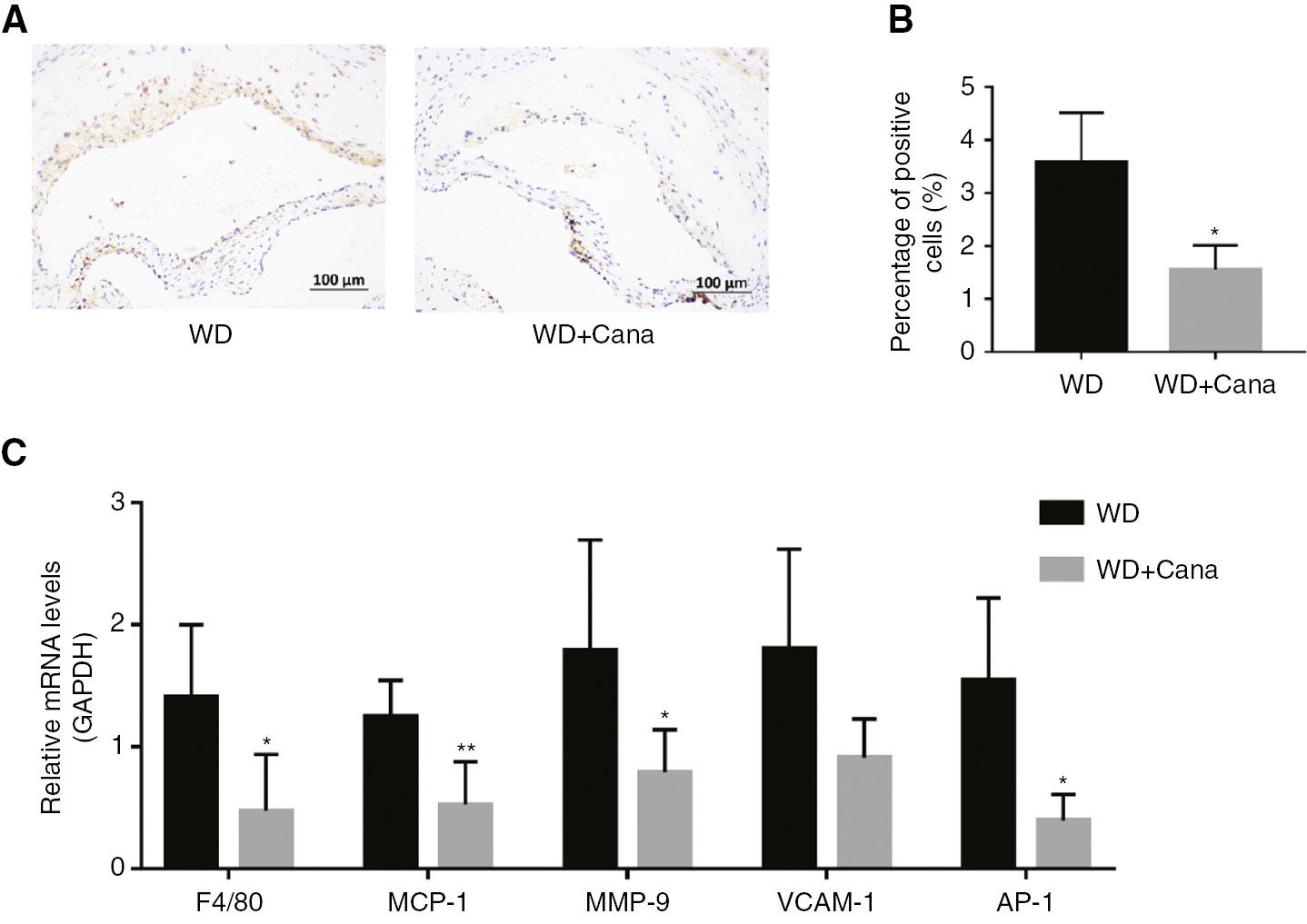
Canagliflozin Mitigates Aortic Inflammation in ApoE−/− Mice.
(A) F4/80, determined by immunohistochemistry (×200 magnification); anti-F4/80: 1/800. (B) Percentage of F4/80 positive cells in the aortic root plaque area (n = 4). (C) Relative mRNA levels of F4/80, MCP-1, MMP-9, VCAM-1, and AP-1. n = 5; *,**P < 0.05, P < 0.01 versus the WD group.
Canagliflozin Ameliorates Aortic Oxidative Stress in ApoE−/− Mice
Oxidative stress is closely associated with aortic inflammation in ApoE−/− mice. We observed ROS levels in aortic roots through ROS staining. A lower areal density of ROS in the aortic roots was observed in mice in the WD+Cana group than the WD group (P = 0.069, Figure 6A, B). To further investigate the effect of canagliflozin on oxidative stress in the aorta, we examined the expression of genes closely associated with oxidative stress. As shown in Figure 6C, the mRNA expression of genes promoting ROS arterial oxidative stress and that of NOX4 was lower in the WD+Cana group than the WD group (P < 0.05 and P = 0.085, respectively), whereas the mRNA levels of Nrf2 and GST, which are involved in resisting oxidative stress, were significantly elevated. Oxidative stress also directly affects endothelial function. eNOS is well known to be closely associated with endothelial function. Our data indicated that Cana treatment increased the mRNA expression of eNOS in the aorta (Figure 6C). Together, these results suggested that Cana alleviates aortic oxidative stress and improves endothelial diastolic function.
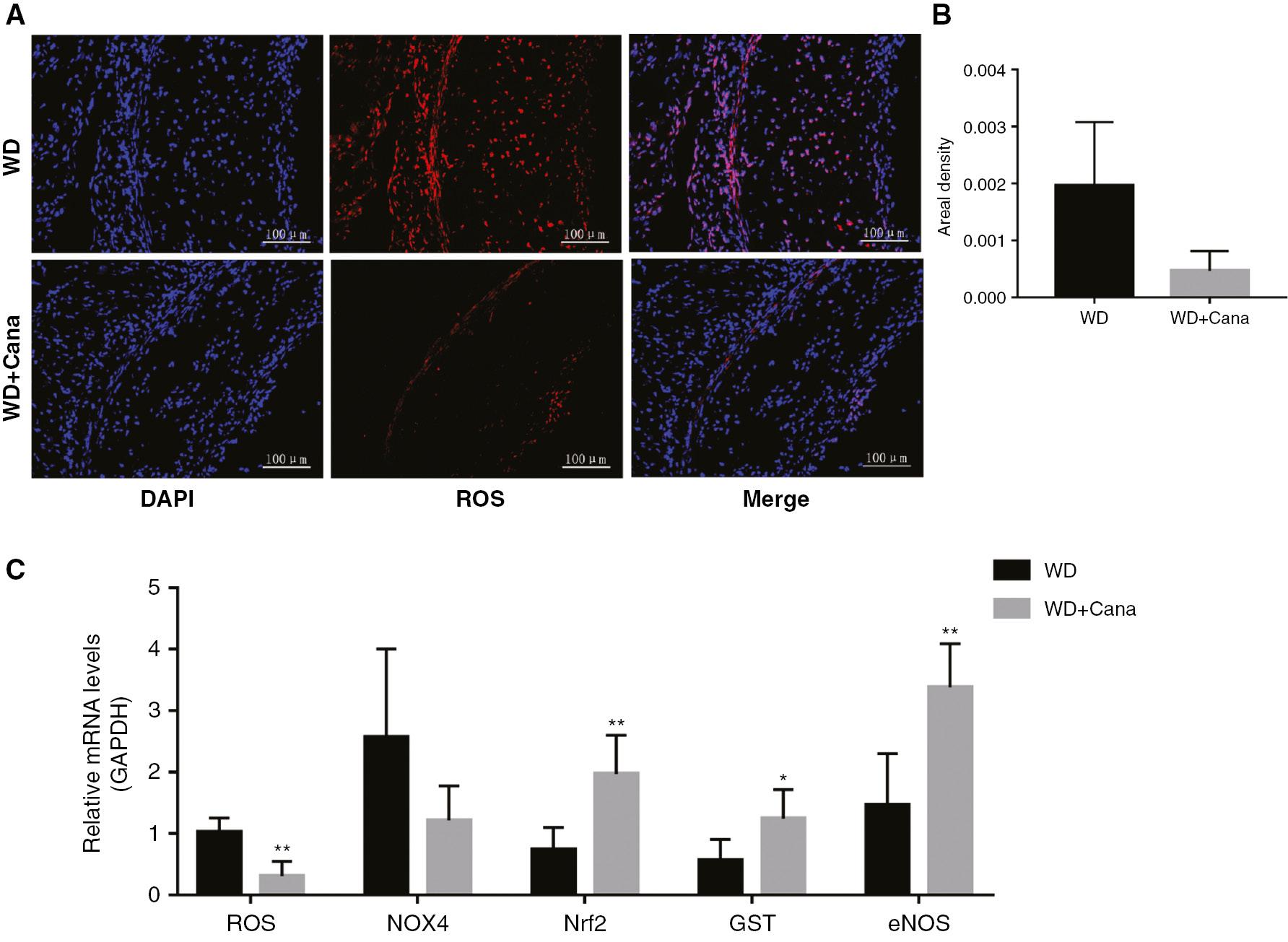
Canagliflozin Alleviates Aortic Oxidative Stress in ApoE−/− Mice.
(A) ROS in the aortic root plaque area, determined by immunofluorescence (×200 magnification); DAPI: λex = 330–380 nm, λem = 420 nm, DCF (red): λex = 488 nm, λem = 525 nm. (B) Relative fluorescence intensity of ROS in aortic root plaques (n = 4). (C) Relative mRNA levels of ROS, NOX4, Nrf2, GST, and eNOS. n = 5; *,**P < 0.05, P < 0.01 versus the WD group.
Canagliflozin Enhances Autophagy in the Aorta in ApoE−/− Mice
Autophagy occurs in all major cell types of atherosclerotic plaques (i.e., macrophages, VSMCs, and ECs) [22, 23]. Autophagy becomes dysfunctional during the development of AS. Therefore, we evaluated the effect of Cana on autophagy in the aorta by detecting expression of the autophagy marker LC3. The relative fluorescence intensity of LC3 in the aortic root in the WD+Cana group was higher than that in the WD group (Figure 7A, B). RT-PCR analysis demonstrated significantly higher expression of LC3B mRNA in the WD+Cana group than the WD group (Figure 7E). Moreover, Cana promoted the formation of autophagosomes. Accumulated P62 and polyubiquitinated proteins co-localize with plaque macrophages [24], thus mediating inflammatory responses, for example through promoting inflammasome formation. Elevated P62 levels in atherosclerotic plaques may reflect abnormal autophagy. In this study, the relative fluorescence intensity of P62 in the aortic root in mice in the WD+Cana group was weaker than that in the WD group (Figure 7C, D), and the mRNA expression of P62 in the aorta was lower than that in the WD group (P < 0.01, Figure 7E). To explore the effect of Cana on autophagy function, we further detected the mRNA expression of NLRP3 and IL-1β, and observed a similar difference, with lower expression in the WD+Cana group (Figure 7E). These results suggested that Cana mediates autophagy in the aorta and subsequent downstream signaling events in ApoE−/− mice.
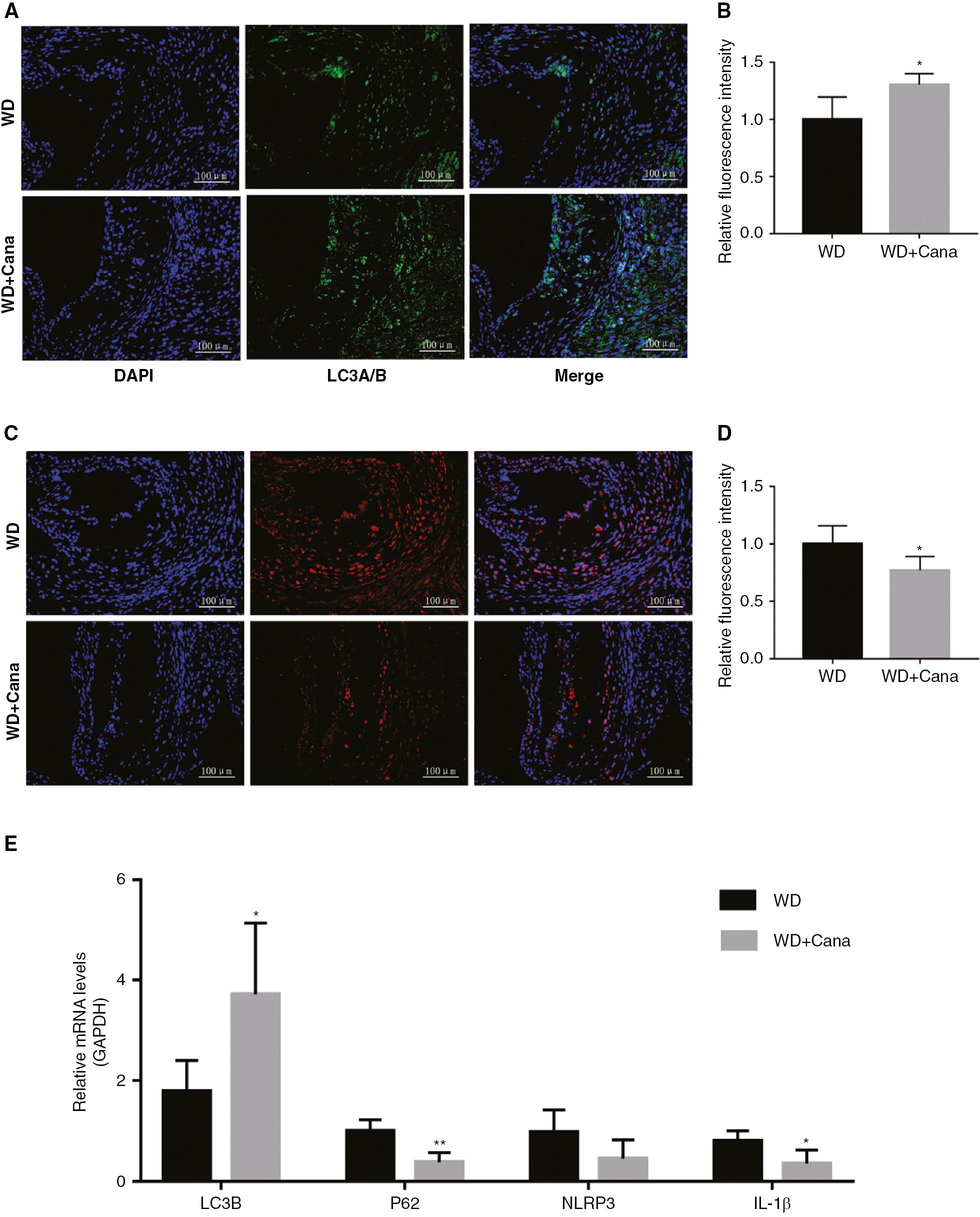
Canagliflozin Enhances Aortic Autophagy in ApoE−/− Mice.
Relative fluorescence intensity of LC3 (A, B) and P62 (C, D) in the aortic root plaque area, determined by immunofluorescence (×200 magnification. n = 4); anti-LC3 A/B: 1/200, and anti-P62: 1/1000; DAPI: λex = 330–380 nm, λem = 420 nm; FITC (green): λex = 492 nm, λem = 520 nm; CY3 (red): λex = 510–560 nm, λem = 590 nm. (E) Relative mRNA levels of LC3B, P62, NLRP3, and IL-1β. n = 5; *,**P < 0.05, P < 0.01 versus the WD group.
Discussion
Diabetes is closely associated with cardiovascular diseases, and ASCVD is the main cause of death and disability in T2DM. SGLT2 inhibitors are a drug of choice in treating patients with T2DM with ASCVD, or those with high risk factors for ASCVD [25]. This study demonstrated Cana’s pleiotropic effects on multiple risk factors, such as obesity, abnormal glucose and lipid metabolism, inflammation, oxidative stress, and autophagy, all of which are closely associated with the occurrence and development of AS.
Moreover, Cana treatment decreased body mass and insulin resistance. After treatment with SGLT2 inhibitors, overweight or obese patients with T2DM have been found to undergo significant weight loss, mainly through the loss of fat mass including visceral fat; this response may potentially associated with an increase in adiponectin levels with SGLT2 inhibitor treatment [26]. In this study, we confirmed that Cana indeed increased the serum level of adiponectin, and determined that weight loss was associated with elevated adiponectin levels. Insulin resistance promotes the formation and progression of AS. SGLT2 inhibitors increase insulin sensitivity in patients with T2DM by decreasing insulin secretion and increasing glucose utilization in tissues [27]. Han et al. [28] have reported that SGLT-2 inhibitors ameliorate AS in high-fat diet-fed ApoE−/− mice, through a mechanism that may partly involve regulation of Akt-GSK-3β pathway to increase insulin sensitivity and decrease insulin resistance.
In this study, Cana improved the lipid profile, through decreasing levels of LDL-C, whereas elevated LDL-C is closely associated with AS. Recent studies have shown that Cana also lowers LDL-C levels by activating AMPK and LXR, and increasing the expression of Abcg5 and Abcg8 in the liver and intestines, thereby increasing cholesterol efflux [10].
To further explore possible anti-AS mechanisms of Cana, we first observed its anti-inflammatory effects, given that inflammation may be critical for AS progression. Numerous studies have shown that inflammatory cells and mediators contribute to the progression of AS, and anti-inflammatory therapy decreases AS risk [29]. Macrophages form foam cells through phagocytosis of oxidized LDL-C, thus generating the lipid core of atherosclerotic plaques. In addition, cytokines secreted by macrophages such as MCP-1 and VCAM-1 may alter the local environment and lead to pathological instability and disease progression [30]. MCP-1 induces the expression of MMP-9 in smooth muscle cells, thus resulting in degradation of the extracellular matrix; therefore, MCP-1 is a key factor in plaque instability [31]. In this study, Cana significantly decreased the serum levels of inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, decreased the infiltration of macrophages in plaques, and inhibited the mRNA expression of cytokines such as F4/80, MCP-1, VCAM-1, and MMP-9. Therefore, Cana may attenuate the progression of AS by decreasing systemic and local inflammation. To further explore the possible anti-inflammatory mechanism of Cana, we examined the gene expression of the upstream transcription factor AP-1 in the aorta. Our findings suggested that Cana may attenuate the inflammatory response mediated by AP-1. However, AP-1 is regulated by JNK and p38 MAPK. Therefore, we speculated that Cana’s effects on the inflammatory response might occur through inhibition of JNK or p38 MAPK signaling molecules.
We observed that Cana alleviated systemic oxidative stress in mice. AS is no longer considered a chronic inflammatory disease characterized solely by dyslipidemia; instead, oxidative stress is also understood to play an important role. SGLT2 inhibitors have been shown to act as antioxidants that ameliorate oxidative stress in the heart [32]. Advanced glycation endproducts transmit signals through their specific receptors and subsequently activate ROS. Many enzymes are involved in the production of ROS, such as NOX; NOX4 is a NOX subtype specific to the vascular system and is significantly elevated in AS [19, 33]. In animal studies, empagliflozin significantly decreases the levels of advanced glycation end products and their specific receptors [34]. In this study, Cana significantly decreased the expression of genes modulating ROS, and possibly that of NOX4, in the aorta. ROS not only oxidize LDL-C but also promote several atherogenic effects, including changes in inflammation and vasodilatory function [35]. To further explore the relevant mechanisms, we examined the gene expression of Nrf2, which acts downstream of ROS in the aorta. Nrf2, an important transcription factor regulating cellular oxidative stress, mediates the expression of a series of constitutive and inducible antioxidant proteins in the immune system. Nrf2 is activated by oxidative stress or electrophilic compounds, and subsequently activates target genes such as GST [36]. In addition, Nrf2 may protect endothelial function by increasing eNOS expression [37]. The mRNA expression of Nrf2, GST, and eNOS in the aorta significantly increased after Cana intervention for 15 weeks. Thus, the ROS/Nrf2 signaling pathway may be a novel target for treatment with Cana.
Finally, we explored the effect of Cana on autophagy. Previous studies have shown that oxidative stress causes protein damage, formation of toxic protein oligomers, protein aggregation, and accumulation of oxidized cellular components. In cells under oxidative stress, autophagy is the main mechanism for removing toxic elements that cannot be degraded by proteasomes; consequently, autophagy is activated by oxidative stress to protect cells from apoptosis [38]. In addition, mitochondrial autophagy eliminates mitochondria damaged by oxidative stress. In this way, the autophagy mechanism eliminates not only damaged mitochondria but also a major source of potentially damaging ROS, whereas impaired autophagy can lead to oxidative stress [39]. The autophagy process itself can be considered a holistic antioxidant mechanism. Autophagy combats atherosclerotic lesions by hydrolyzing cholesterol deposits in macrophages, preventing plaque formation, and inhibiting inflammatory responses [40, 41]. The inflammasome is a cytoplasmic protein complex that leads to the processing and subsequent release of IL-1β, and NLRP3 is a key component of the inflammasome. ROS and lysosome damage induce inflammasome activation, but this process is inhibited by autophagy clearance of damaged organelles [42]. Our findings suggested that Cana may decrease oxidative stress and inflammation through autophagy, and may have a role in preventing AS.
Conclusions
This study indicated that Cana improves glucose and lipid metabolism, and decreases systemic and aortic local inflammation, as well as oxidative stress, in ApoE−/− mice fed a WD. We further showed that Cana may decrease the inflammatory response mediated by AP-1 and oxidative stress through the ROS/Nrf2 pathway. In addition, our results suggested that Cana enhances autophagy and thus may have anti-AS effects. Nevertheless, additional in vitro studies are recommended to verify our proposed mechanisms. In future work, our methods could be improved, for example, by evaluating the tissue distribution of Cana; varying the dose and route of drug administration; and adding two control groups: one with C57BL/6J mice and one with SGLT2-knockout ApoE−/− mice. In addition, to verify our results, additional clinical research is recommended. In future research, we plan to explore specific inflammatory pathways and autophagy regulatory mechanisms of SGLT2 inhibitors.