- Record: found
- Abstract: found
- Article: found
Mutations within FGFR1 are associated with superior outcome in a series of 83 diffuse midline gliomas with H3F3A K27M mutations
brief-report

Ulrich Schüller
1
,
2
,
3
,
,
Peter Iglauer
4 ,
Mario M. Dorostkar
5
,
6 ,
Christian Mawrin
7 ,
Jochen Herms
5
,
6 ,
Armin Giese
5 ,
Markus Glatzel
1 ,
Julia E. Neumann
1
,
12 January 2021
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Diffuse midline glioma (DMG), H3 K27M mutant (WHO grade IV) is listed as a separate
CNS tumor entity since 2016 [5], after large sequencing efforts had discovered H3
K27M mutations frequently appearing in gliomas located in midline structures [11].
Over time, we and others have observed single cases of DMG with concomitant mutations
within FGFR1 or BRAF [1, 2, 4, 6, 7, 9, 10, 12–14]. FGFR1 and BRAF mutations are typical
hallmarks of low grade glioma, such as pilocytic astrocytoma, ganglioglioma, or dysembryoplastic
neuroepithelial tumor [3, 8]. So, the parallel occurrence of H3 and FGFR1/BRAF mutations
within a single tumor may complicate the diagnostic decision towards a low grade or
a high grade glioma. This dilemma, which has direct clinical implications, is particularly
evident, if only small biopsies are taken and low-grade histology may not be respresentative
and hence may not mirror the biology of the neoplasm. On the other hand, the presence
of a MAPK pathway alteration, such as FGFR1 or BRAF mutations, may open up additional
possibilities of targeted therapies, independent of the tumor classification.
In order to learn more about the frequency and impact on such mutations, we analyzed
a series of 83 DMG, H3F3A K27M mutant. Details on clinical characteristics of patients
are listed in Fig. 1a and Supplementary Table 1, online resource. One case (1.2%)
displayed a BRAF (p.V600E) mutation and 9/83 cases (10.8%) showed FGFR1 mutations
(p.K656E or p.N546K). Mutations within NF1, TP53, and ATRX were detected in 31.8%,
51.4%, and 35.2%, respectively. TP53 mutations were significantly associated with
FGFR1 wild type status (FGFR1 WT, p = 0.009, Χ
2-test, Supplementary Fig. 1a, online resource).
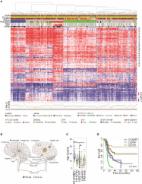
Fig. 1
Clinical, histological, and molecular parameters of H3F3A K27M mutated DMG with and
without additional mutations in FGFR1. a Overview on all 83 analyzed cases with 12%
of cases harboring BRAF or FGFR1 hotspot mutations. Percentages of characteristics
refer to cases with known attribute only. Representative images of FGFR1 WT (b, c)
and MU cases (d, e) demonstrate comparable histomorphology in both groups. T-SNE analysis
of DMG reveals FGFR1 and BRAF MU cases to harbor similar DNA methylation profiles
as FGFR1 and BRAF WT cases (f). FGFR1 MU cases showed a significantly better prognosis
than FGFR1 WT cases (p = 0.023, g), and multivariate analyses confirmed significance
of FGFR1 status independent of age and localization. WT* = wild type for respective
hotspot, MU = mutant, n. a. = not available, *WHO grade of initial diagnosis
Similar to FGFR1 WT cases, cases with additional FGFR1 mutation displayed features
of a diffusely growing glioma with increased cellularity and signs of anaplasia, such
as increased cell pleomorphism, mitoses, or vessel proliferation (Fig. 1b-e). Furthermore,
all analyzed FGFR1 MU cases (and the BRAF MU case) matched to the methylation class”DMG,
H3 K27M mutant” (Supplementary Fig. 1b, online resource, Fig. 1f, Supplementary Table
1, online resource).
Higher age (≥ 18 years), supratentorial tumor localization and FGFR1 MU status were
associated with a significantly better prognosis of patients (p = 0.038, p = 0.034,
and p = 0.023, Fig. 1g and Supplementary Fig. 2a, b, online resource). In contrast,
TP53 MU status was associated with a significantly worse prognosis of patients (p = 0.002,
Supplementary Fig. 2c, online resource). Including the latter factors in a multivariate
cox regression analyses showed localization and TP53 status as significant variables
(Supplementary Fig. 2d, online resource). FGFR1 and TP53 mutations occurred almost
mutually exclusive and hence did not represent independent variables (see also Supplementary
Fig. 1a, online resource). Thus, we performed a multivariate analysis including the
independent variables age, localization, and FGFR1 status only (Fig. 1h). In this
context, FGFR1 MU status was significantly associated with a better overall survival,
independently of patient age, and tumor localization (p = 0.026). Interestingly, the
single patient (#56) with an accompanying BRAF p.V600E mutation remained alive at
24.5 months after initial diagnosis. However, the prognosis for such diffuse midline
gliomas with dual H3F3A p.K27M and BRAF p.V600E mutations remains to be defined.
Together, our results suggest that RAS-MAPK-pathway signaling might play an important
role in DMG with implications for diagnosis, prognosis, and therapy of respective
patients.
Supplementary Information
Below is the link to the Supplementary Information.
Supplementary file 1 (PPTX 3817 kb)
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma.
- Record: found
- Abstract: found
- Article: found
Integrated Molecular Meta-Analysis of 1,000 Pediatric High-Grade and Diffuse Intrinsic Pontine Glioma
Alan Mackay, Anna Burford, Diana Carvalho … (2017)
- Record: found
- Abstract: found
- Article: not found
Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma.
Benjamin Raeder, Scott Pomeroy, Charles D Imbusch … (2013)