- Record: found
- Abstract: found
- Article: found
Establishment of a bi-layered tissue engineered conjunctiva using a 3D-printed melt electrowritten poly-(ε-caprolactone) scaffold
Read this article at
Abstract
Purpose
To utilize melt electrowriting (MEW) technology using poly-(ε-caprolactone) (PCL) coupled with a 2-step co-culturing strategy for the development of a conjunctival bi-layer synthetic construct.
Methods
Melt electrowritten scaffolds using PCL were fabricated using an in-house-built MEW printer. Human conjunctival stromal cells (CjSCs) and epithelial cells (CjECs) were isolated from donor tissue. A 2-step co-culture method was done by first seeding the CjSCs and culturing for 4 weeks to establish a stromal layer, followed by CjECs and co-culturing for 2 more weeks. Cultured cells were each characterized by morphology and marker expression on immunofluorescence and qPCR. The produced construct was assessed for cellular proliferation using viability assays. The bi-layer morphology was assessed using scanning electron microscopy (SEM), confocal microscopy, and immunofluorescence imaging. The expression of extracellular matrix components and TGF-b was evaluated using qPCR.
Results
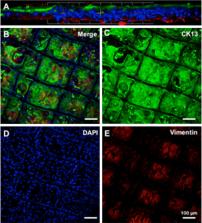
CjSCs were spindle-shaped and vimentin + while CjECs were polygonal and CK13 + . CjSCs showed consistent proliferation and optimal adherence with the scaffold at the 4-week culture mark. A 2-layered construct consisting of a CjSC-composed stromal layer and a CjEC-composed epithelial layer was appreciated on confocal microscopy, SEM, and immunofluorescence. CjSCs secreted collagens (types I, V, VI) but at differing amounts from natural tissue while TGF-b production was comparable.
Conclusion
The 3D-printed melt electrowritten PCL scaffold paired with the 2-step co-culturing conditions of the scaffold allowed for the first approximation of a bi-layered stromal and epithelial reconstruction of the conjunctiva that can potentially improve the therapeutic arsenal in ocular surface reconstruction.
Related collections
Most cited references43
- Record: found
- Abstract: not found
- Article: not found
The return of a forgotten polymer—Polycaprolactone in the 21st century
- Record: found
- Abstract: found
- Article: not found
Direct writing by way of melt electrospinning.
- Record: found
- Abstract: found
- Article: not found