- Record: found
- Abstract: found
- Article: found
Murine Polyomavirus Virus-Like Particles Carrying Full-Length Human PSA Protect BALB/c Mice from Outgrowth of a PSA Expressing Tumor
Read this article at
Abstract
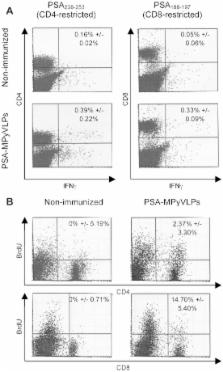
Virus-like particles (VLPs) consist of capsid proteins from viruses and have been shown to be usable as carriers of protein and peptide antigens for immune therapy. In this study, we have produced and assayed murine polyomavirus (MPyV) VLPs carrying the entire human Prostate Specific Antigen (PSA) (PSA-MPyVLPs) for their potential use for immune therapy in a mouse model system. BALB/c mice immunized with PSA-MPyVLPs were only marginally protected against outgrowth of a PSA-expressing tumor. To improve protection, PSA-MPyVLPs were co-injected with adjuvant CpG, either alone or loaded onto murine dendritic cells (DCs). Immunization with PSA-MPyVLPs loaded onto DCs in the presence of CpG was shown to efficiently protect mice from tumor outgrowth. In addition, cellular and humoral immune responses after immunization were examined. PSA-specific CD4 + and CD8 + cells were demonstrated, but no PSA-specific IgG antibodies. Vaccination with DCs loaded with PSA-MPyVLPs induced an eight-fold lower titre of anti-VLP antibodies than vaccination with PSA-MPyVLPs alone. In conclusion, immunization of BALB/c mice with PSA-MPyVLPs, loaded onto DCs and co-injected with CpG, induces an efficient PSA-specific tumor protective immune response, including both CD4 + and CD8 + cells with a low induction of anti-VLP antibodies.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic.
- Record: found
- Abstract: found
- Article: not found
A controlled trial of leuprolide with and without flutamide in prostatic carcinoma.
- Record: found
- Abstract: found
- Article: not found
Phase I trial of yttrium-90-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.