- Record: found
- Abstract: found
- Article: found
Stabilizing lithium metal anode by octaphenyl polyoxyethylene-lithium complexation
Read this article at
Abstract
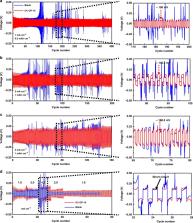
Lithium metal is an ideal anode for lithium batteries due to its low electrochemical potential and high theoretical capacity. However, safety issues arising from lithium dendrite growth have significantly reduced the practical applicability of lithium metal batteries. Here, we report the addition of octaphenyl polyoxyethylene as an electrolyte additive to enable a stable complex layer on the surface of the lithium anode. This surface layer not only promotes uniform lithium deposition, but also facilitates the formation of a robust solid-electrolyte interface film comprising cross-linked polymer. As a result, lithium|lithium symmetric cells constructed using the octaphenyl polyoxyethylene additive exhibit excellent cycling stability over 400 cycles at 1 mA cm −2, and outstanding rate performance up to 4 mA cm −2. Full cells assembled with a LiFePO 4 cathode exhibit high rate capability and impressive cyclability, with capacity decay of only 0.023% per cycle.
Abstract
Despite the large theoretical promise of Li metal anode, the dendrite growth poses a serious safety challenge. Here the authors address this issue by adding octaphenyl polyoxyethylene as an electrolyte additive which facilitates the formation of a dual-functional layer and excellent performance.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries.
- Record: found
- Abstract: found
- Article: not found