- Record: found
- Abstract: found
- Article: found
Complexity and multi-factoriality of Trypanosoma cruzi sylvatic cycle in coatis, Nasua nasua (Procyonidae), and triatomine bugs in the Brazilian Pantanal
Read this article at
Abstract
Background
Trypanosoma cruzi is dispersed in nature through many transmission mechanisms among a high diversity of vectors and mammalian species, representing particular behaviors and habitats. Thus, each locality has a unique set of conditions underlying the maintenance of this parasite in the wild. The aim of the present study was to evaluate the life-cycle of T. cruzi in free-ranging coatis from the central region of the Brazilian Pantanal using a multi-factorial approach.
Methods
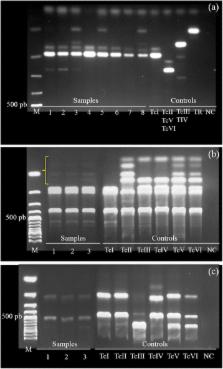
Three methodological blocks were used in the present study: (i) We evaluated T. cruzi infection using serological (ELISA) and parasitological (hemoculture) tests in free-ranging coatis captured from October 2010 to March 2012. In addition, we characterized T. cruzi isolates as DTUs (Discrete Typing Units); (ii) We evaluated Trypanosoma infection in species of Triatoma and Rhodnius inhabiting coati arboreal nests from May to September 2012 using parasitological and molecular assays; and (iii) We analyzed a set of longitudinal data (from 2005 to 2012) concerning the effects of T. cruzi infection on this coati population. Herein, we investigated whether seasonality, host sex, and host age influence T. cruzi prevalence and patterns of infection.
Results
The 2010–2012 period presented high seroprevalence on coatis (72.0 % ELISA) and a high percentage of individuals with infectivity competence (20.5 % positive hemoculture). All isolates presented TcI band patterns, occurring in single ( n = 3) and mixed infections (1 TcI/ T. rangeli; 4 with undefined characterization). Both male and female individuals presented the same transmission potential, expressed as positive hemoculture, which was only detected in the summer. However, overall, the data (2005–2012) highlighted the importance of females for T. cruzi maintenance in the winter. Moreover, twenty-three (67.6 %) bugs from five coati nests (71.4 %) were infected with flagellated forms. Seventeen samples were characterized as T. cruzi (TcI and TcIII genotypes).
Conclusion
In the Pantanal region, T. cruzi is transmitted in a complex, multifactorial, dynamic and non-linear transmission web. The coati nests might be inserted in this web, acting as important transmission foci at the arboreal stratum to different mammal species with arboreal or scansorial behavior.
Related collections
Most cited references44
- Record: found
- Abstract: not found
- Book: not found
Molecular Cloning : A Laboratory Manual
- Record: found
- Abstract: found
- Article: not found
Mechanism of genetic exchange in American trypanosomes.
- Record: found
- Abstract: found
- Article: not found