- Record: found
- Abstract: found
- Article: found
Adaptive NK cells in people exposed to Plasmodium falciparum correlate with protection from malaria
Read this article at
Abstract
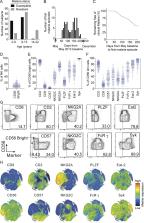
Plasmodium falciparum–infected individuals in a malaria-endemic region have an abundance of adaptive NK cells that correlates with resistance to malaria. Adaptive NK cells dominate antibody-dependent responses to P. falciparum–infected red blood cells and may contribute to acquired immunity to malaria.
Abstract
How antibodies naturally acquired during Plasmodium falciparum infection provide clinical immunity to blood-stage malaria is unclear. We studied the function of natural killer (NK) cells in people living in a malaria-endemic region of Mali. Multi-parameter flow cytometry revealed a high proportion of adaptive NK cells, which are defined by the loss of transcription factor PLZF and Fc receptor γ-chain. Adaptive NK cells dominated antibody-dependent cellular cytotoxicity responses, and their frequency within total NK cells correlated with lower parasitemia and resistance to malaria. P. falciparum–infected RBCs induced NK cell degranulation after addition of plasma from malaria-resistant individuals. Malaria-susceptible subjects with the largest increase in PLZF-negative NK cells during the transmission season had improved odds of resistance during the subsequent season. Thus, antibody-dependent lysis of P. falciparum–infected RBCs by NK cells may be a mechanism of acquired immunity to malaria. Consideration of antibody-dependent NK cell responses to P. falciparum antigens is therefore warranted in the design of malaria vaccines.
Related collections
Most cited references22
- Record: found
- Abstract: not found
- Article: not found
Gamma-globulin and acquired immunity to human malaria.
- Record: found
- Abstract: found
- Article: not found
Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function.
- Record: found
- Abstract: not found
- Article: not found