- Record: found
- Abstract: found
- Article: not found
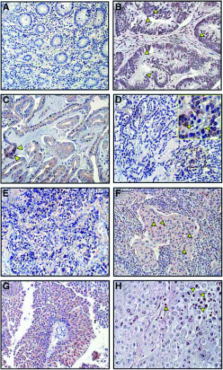
Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted
Read this article at
Abstract
Tumours ferment glucose to lactate even in the presence of oxygen (aerobic glycolysis; Warburg effect). The pentose phosphate pathway (PPP) allows glucose conversion to ribose for nucleic acid synthesis and glucose degradation to lactate. The nonoxidative part of the PPP is controlled by transketolase enzyme reactions. We have detected upregulation of a mutated transketolase transcript (TKTL1) in human malignancies, whereas transketolase (TKT) and transketolase-like-2 (TKTL2) transcripts were not upregulated. Strong TKTL1 protein expression was correlated to invasive colon and urothelial tumours and to poor patients outcome. TKTL1 encodes a transketolase with unusual enzymatic properties, which are likely to be caused by the internal deletion of conserved residues. We propose that TKTL1 upregulation in tumours leads to enhanced, oxygen-independent glucose usage and a lactate-based matrix degradation. As inhibition of transketolase enzyme reactions suppresses tumour growth and metastasis, TKTL1 could be the relevant target for novel anti-transketolase cancer therapies. We suggest an individualised cancer therapy based on the determination of metabolic changes in tumours that might enable the targeted inhibition of invasion and metastasis.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
AMP-activated protein kinase induces a p53-dependent metabolic checkpoint.
- Record: found
- Abstract: found
- Article: not found
Akt stimulates aerobic glycolysis in cancer cells.
- Record: found
- Abstract: found
- Article: not found
