- Record: found
- Abstract: not found
- Article: not found
Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus
August 26 2018
August 26 2018
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
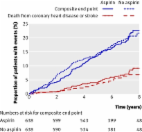
Diabetes mellitus is associated with an increased risk of cardiovascular events. Aspirin
use reduces the risk of occlusive vascular events but increases the risk of bleeding;
the balance of benefits and hazards for the prevention of first cardiovascular events
in patients with diabetes is unclear.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies.
Enrico Flossmann, Peter M. Rothwell (2007)
- Record: found
- Abstract: found
- Article: found
The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease
Jill Belch, Angus MacCuish, Iain Campbell … (2008)
- Record: found
- Abstract: found
- Article: not found
Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial.
Hisao Ogawa, Masafumi Nakayama, Takeshi Morimoto … (2008)
