- Record: found
- Abstract: found
- Article: found
Brain Kynurenine and BH4 Pathways: Relevance to the Pathophysiology and Treatment of Inflammation-Driven Depressive Symptoms
Read this article at
Abstract
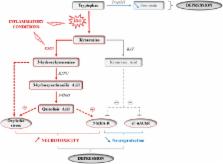
The prevalence of depressive disorders is growing worldwide, notably due to stagnation in the development of drugs with greater antidepressant efficacy, the continuous large proportion of patients who do not respond to conventional antidepressants, and the increasing rate of chronic medical conditions associated with an increased vulnerability to depressive comorbidities. Accordingly, better knowledge on the pathophysiology of depression and mechanisms underlying depressive comorbidities in chronic medical conditions appears urgently needed, in order to help in the development of targeted therapeutic strategies. In this review, we present evidence pointing to inflammatory processes as key players in the pathophysiology and treatment of depressive symptoms. In particular, we report preclinical and clinical findings showing that inflammation-driven alterations in specific metabolic pathways, namely kynurenine and tetrahydrobiopterin (BH4) pathways, leads to substantial alterations in the metabolism of serotonin, glutamate and dopamine that are likely to contribute to the development of key depressive symptom dimensions. Accordingly, anti-inflammatory interventions targeting kynurenine and BH4 pathways may be effective as novel treatment or as adjuvants of conventional medications rather directed to monoamines, notably when depressive symptomatology and inflammation are comorbid in treated patients. This notion is discussed in the light of recent findings illustrating the tight interactions between known antidepressant drugs and inflammatory processes, as well as their therapeutic implications. Altogether, this review provides valuable findings for moving toward more adapted and personalized therapeutic strategies to treat inflammation-related depressive symptoms.
Related collections
Most cited references158
- Record: found
- Abstract: found
- Article: not found
Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure.
- Record: found
- Abstract: found
- Article: not found
Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression.
- Record: found
- Abstract: found
- Article: not found