- Record: found
- Abstract: found
- Article: found
A comprehensive, longitudinal analysis of humoral responses specific to four recombinant antigens of SARS-CoV-2 in severe and non-severe COVID-19 patients
Abstract
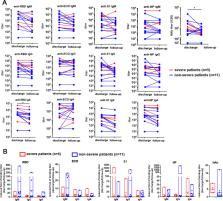
There is an urgent need for effective treatment and preventive vaccine to contain this devastating global pandemic, which requires a comprehensive understanding of humoral responses specific to SARS-CoV-2 during the disease progression and convalescent phase of COVID-19 patients. We continuously monitored the serum IgM and IgG responses specific to four SARS-CoV-2 related antigens, including the nucleoprotein (NP), receptor binding domain (RBD), S1 protein, and ectodomain (ECD) of the spike protein among non-severe and severe COVID-19 patients for seven weeks since disease onset. Most patients generated humoral responses against NP and spike protein-related antigens but with their distinct kinetics profiles. Combined detection of NP and ECD antigens as detecting antigen synergistically improved the sensitivity of the serological assay, compared to that of using NP or RBD as detection antigen. 80.7% of convalescent sera from COVID-19 patients revealed that the varying extents of neutralization activities against SARS-CoV-2. S1-specific and ECD-specific IgA responses were strongly correlated with the neutralization activities in non-severe patients, but not in severe patients. Moreover, the neutralizing activities of the convalescent sera were shown to significantly decline during the period between 21 days to 28 days after hospital discharge, accompanied by a substantial drop in RBD-specific IgA response. Our data provide evidence that are crucial for serological testing, antibody-based intervention, and vaccine design of COVID-19.
Author summary
The world is facing an unprecedented challenge with communities and economies affected by the growing pandemic of coronavirus disease 2019 (COVID-19). Currently, there is no vaccine or effective drugs have been approved to treat or prevent COVID-19. The development of antibody response to SARS-CoV-2, the virus that causes COVID-19, started to be reported but remained largely elusive. Understanding the adaptive responses where the body makes antibodies that specifically bind to the SARS-CoV-2 among COVID-19 patients provides fundamental information for developing effective treatment and preventive vaccine. In this study, we not only successively analyzed the specificity and magnitude of antibody responses using four SARS-CoV-2 related antigens, but also monitored the neutralization potency of the convalescent sera from COVID-19 patients at the time point of hospital discharge and follow-up visit. Our results indicated that most patients generated humoral responses against nucleoprotein and three spike protein-related antigens with their distinct kinetics profiles. Additionally, most convalescent sera had the varying extents of neutralization activities against SARS-CoV-2. Of note, we identified that IgA antibody responses specific to S1 and ECD were strongly correlated with neutralization activities in non-severe patients, but not in severe patients. Furthermore, we identified a significant reduction of neutralizing activities of the convalescent sera within one month. Our data provide a collective basis of serological testing, antibody-based intervention, and vaccine design of COVID-19.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Clinical Characteristics of Coronavirus Disease 2019 in China
- Record: found
- Abstract: found
- Article: not found
Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study
- Record: found
- Abstract: found
- Article: not found