- Record: found
- Abstract: found
- Article: found
Quality assessment of mobile applications on postpartum hemorrhage management Translated title: Evaluación de la calidad de aplicaciones móviles en el manejo de la hemorragia posparto Translated title: Avaliação da qualidade de aplicativos móveis sobre o manejo da hemorragia pós-parto
ABSTRACT
Objective:
To assess mobile application quality on the management of postpartum hemorrhage available in the digital stores of the main operating systems.
Method:
A descriptive evaluative study, carried out from January to February 2023 on the App Store ® and Google Play Store ®. The Mobile Application Rating Scale was used to assess quality (engagement, functionality, aesthetics, information and subjective quality). Information extraction and assessment on postpartum hemorrhage was carried out using a table with information based on official documents, containing stratification, prevention, diagnosis and treatment.
Results:
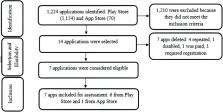
Seven applications were included; of these, three were in English, six had an Android operating system. The quality mean was 3.88. The highest means were for functionality, reaching 5.0 (n = 6), and the lowest were for engagement, less than 3.0 (n = 4). The majority of applications presented less than 50% of the information on postpartum hemorrhage management.
RESUMO
Avaliar a qualidade dos aplicativos móveis sobre o manejo da hemorragia pós-parto disponíveis nas lojas digitais dos principais sistemas operacionais.
Estudo descritivo de avaliação, realizado de janeiro a fevereiro de 2023 nas lojas digitais App Store® e Google Play Store®. Foi utilizada a Mobile Application Rating Scale para avaliação da qualidade (engajamento, funcionalidade, estética, informação e qualidade subjetiva). A extração e a avaliação das informações sobre hemorragia pós-parto foram realizadas a partir de um quadro com informações baseadas em documentos oficiais, contendo a classificação, prevenção, diagnóstico e tratamento.
Sete aplicativos foram incluídos; desses, três estavam em inglês, seis tinham sistema operacional Android. A média de qualidade foi de 3,88. As maiores médias foram da funcionalidade, alcançando 5,0 (n = 6), e as menores foram de engajamento, menos que 3,0 (n = 4). A maioria dos aplicativos apresentou menos de 50% das informações sobre o manejo de hemorragia pós-parto.
RESUMEN
Evaluar la calidad de las aplicaciones móviles sobre el manejo de la hemorragia posparto disponibles en las tiendas digitales de los principales sistemas operativos.
Estudio de evaluación descriptivo, realizado de enero a febrero de 2023 en las tiendas digitales App Store ® y Google Play Store ®. Se utilizó la Escala de Calificación de Aplicaciones Móviles para evaluar la calidad (compromiso, funcionalidad, estética, información y calidad subjetiva). La extracción y evaluación de la información sobre la hemorragia posparto se realizó mediante una tabla con información basada en documentos oficiales, que contiene clasificación, prevención, diagnóstico y tratamiento.
Se incluyeron siete aplicaciones; de ellos, tres estaban en inglés, seis tenían sistema operativo Android. El promedio de calidad fue 3,88. Los promedios más altos fueron para la funcionalidad, alcanzando 5,0 (n = 6), y los más bajos fueron para el compromiso, menos de 3,0 (n = 4). La mayoría de las solicitudes presentaron menos del 50% de la información sobre el manejo de la hemorragia posparto.
Related collections
Most cited references33

- Record: found
- Abstract: found
- Article: found
PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews
- Record: found
- Abstract: found
- Article: found
Mobile App Rating Scale: A New Tool for Assessing the Quality of Health Mobile Apps
- Record: found
- Abstract: found
- Article: found