- Record: found
- Abstract: found
- Article: found
Aggressive Locoregional Treatment Improves the Outcome of Liver Metastases from Grade 3 Gastroenteropancreatic Neuroendocrine Tumors
Read this article at
Abstract
Grade 3 (G3) gastroenteropancreatic (GEP) neuroendocrine tumors (NETs) are rare, and there is no report specifically dealing with patients of liver metastases from G3 GEP NETs.
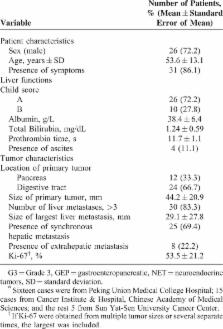
From January 2004 to January 2014, 36 conservative patients with G3 GEP NET liver metastases were retrospectively identified from 3 hepatobiliary centers in China. The clinical features and treatment outcomes were analyzed.
Aggressive locoregional treatments (LT, including cytoreductive surgery, radiofrequency ablation, and liver-directed intra-arterial intervention) and systemic therapy (ST) were introduced separately or combined, with 26 (72%) patients receiving resection of primary tumor and/or hepatic metastases, 12 patients receiving non-surgical locoregional interventions (NSLRIs), and 22 patients receiving certain kind of STs. Median overall survival (OS) was 20.0 months (95% confidence interval [CI]: 8.9–31.1 months) and survival rates were 62.6%, 30.1%, and 19.8%, at 1, 3, and 5 years, respectively. The median OS was 9.0 months (95%CI: 3.3–14.7 months) for patients receiving only STs (n = 6), 19 months (95%CI: 1.3–36.8 months) for patients receiving LT followed by STs (n = 16), and 101 months (95%CI: 0.0–210.2 months) for patients receiving only LT (n = 12). Moreover, compared with those receiving only ST or best supportive care, patients given certain types of LTs had higher rates of symptom alleviation (3/8 versus 20/23). On univariate analysis, positive prognostic factors of survival were pancreatic primary tumor ( P = 0.013), normal total bilirubin level ( P = 0.035), receiving surgery ( P = 0.034), receiving NSLRI ( P = 0.014), and sum of diameters of remnant tumor < 5 cm ( P = 0.008). On multivariate analyses, pancreatic primary tumor ( P = 0.015), normal total bilirubin level ( P = 0.002), and sum of diameters of remnant tumor < 5 cm ( P = 0.001) remained to be independent prognostic factors.
For patients with G3 GEP NET liver metastases, aggressive LTs may improve clinical outcomes. Larger studies with prospective design are warranted to consolidate these results, and to discover the most appropriate seletion criteria for patients to undergo different kinds of aggressive LTs and to find the most effective combinations, with or without ST.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study.
- Record: found
- Abstract: found
- Article: not found
The epidemiology of gastroenteropancreatic neuroendocrine tumors.
- Record: found
- Abstract: found
- Article: not found