- Record: found
- Abstract: found
- Article: found
Role of putative voltage-sensor countercharge D4 in regulating gating properties of Ca V1.2 and Ca V1.3 calcium channels
Read this article at
ABSTRACT
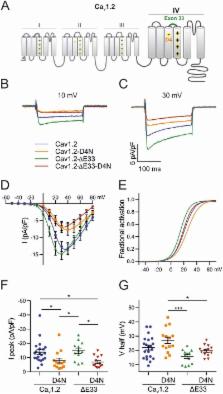
Voltage-dependent calcium channels (Ca V) activate over a wide range of membrane potentials, and the voltage-dependence of activation of specific channel isoforms is exquisitely tuned to their diverse functions in excitable cells. Alternative splicing further adds to the stunning diversity of gating properties. For example, developmentally regulated insertion of an alternatively spliced exon 29 in the fourth voltage-sensing domain (VSD IV) of Ca V1.1 right-shifts voltage-dependence of activation by 30 mV and decreases the current amplitude several-fold. Previously we demonstrated that this regulation of gating properties depends on interactions between positive gating charges (R1, R2) and a negative countercharge (D4) in VSD IV of Ca V1.1. Here we investigated whether this molecular mechanism plays a similar role in the VSD IV of Ca V1.3 and in VSDs II and IV of Ca V1.2 by introducing charge-neutralizing mutations (D4N or E4Q) in the corresponding positions of Ca V1.3 and in two splice variants of Ca V1.2. In both channels the D4N (VSD IV) mutation resulted in a ̴5 mV right-shift of the voltage-dependence of activation and in a reduction of current density to about half of that in controls. However in Ca V1.2 the effects were independent of alternative splicing, indicating that the two modulatory processes operate by distinct mechanisms. Together with our previous findings these results suggest that molecular interactions engaging D4 in VSD IV contribute to voltage-sensing in all examined Ca V1 channels, however its striking role in regulating the gating properties by alternative splicing appears to be a unique property of the skeletal muscle Ca V1.1 channel.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Voltage-gated calcium channels.
- Record: found
- Abstract: found
- Article: not found
Structure of the voltage-gated calcium channel Cav1.1 at 3.6 Å resolution.

- Record: found
- Abstract: found
- Article: found