- Record: found
- Abstract: found
- Article: found
Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: from bench to bedside
Read this article at
Abstract
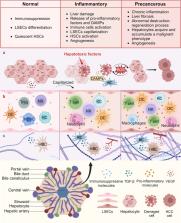
Hepatocellular carcinoma (HCC) is a highly heterogeneous malignancy with high incidence, recurrence, and metastasis rates. The emergence of immunotherapy has improved the treatment of advanced HCC, but problems such as drug resistance and immune-related adverse events still exist in clinical practice. The immunosuppressive tumor microenvironment (TME) of HCC restricts the efficacy of immunotherapy and is essential for HCC progression and metastasis. Therefore, it is necessary to elucidate the mechanisms behind immunosuppressive TME to develop and apply immunotherapy. This review systematically summarizes the pathogenesis of HCC, the formation of the highly heterogeneous TME, and the mechanisms by which the immunosuppressive TME accelerates HCC progression and metastasis. We also review the status of HCC immunotherapy and further discuss the existing challenges and potential therapeutic strategies targeting immunosuppressive TME. We hope to inspire optimizing and innovating immunotherapeutic strategies by comprehensively understanding the structure and function of immunosuppressive TME in HCC.
Related collections
Most cited references371
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
Hallmarks of Cancer: New Dimensions
- Record: found
- Abstract: found
- Article: not found