- Record: found
- Abstract: found
- Article: found
A Generator-Produced Gallium-68 Radiopharmaceutical for PET Imaging of Myocardial Perfusion
Read this article at
Abstract
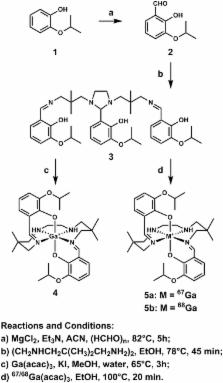
Lipophilic cationic technetium-99m-complexes are widely used for myocardial perfusion imaging (MPI). However, inherent uncertainties in the supply chain of molybdenum-99, the parent isotope required for manufacturing 99Mo/ 99mTc generators, intensifies the need for discovery of novel MPI agents incorporating alternative radionuclides. Recently, germanium/gallium (Ge/Ga) generators capable of producing high quality 68Ga, an isotope with excellent emission characteristics for clinical PET imaging, have emerged. Herein, we report a novel 68Ga-complex identified through mechanism-based cell screening that holds promise as a generator-produced radiopharmaceutical for PET MPI.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs.
- Record: found
- Abstract: found
- Article: not found
P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model.
- Record: found
- Abstract: found
- Article: not found