- Record: found
- Abstract: found
- Article: found
Quantitative Modeling of Escherichia coli Chemotactic Motion in Environments Varying in Space and Time
Read this article at
Abstract
Escherichia coli chemotactic motion in spatiotemporally varying environments is studied by using a
computational model based on a coarse-grained description of the intracellular signaling
pathway dynamics. We find that the cell's chemotaxis drift velocity
v
d
is a constant in an exponential attractant concentration gradient [
L]∝exp(
Gx).
v
d
depends linearly on the exponential gradient
G before it saturates when
G is larger than a critical value
G
C
. We find that
G
C
is determined by the intracellular adaptation rate
k
R
with a simple scaling law:
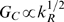 . The linear dependence of
v
d
on
G =
d(ln[
L])/
dx directly demonstrates
E. coli's ability in sensing the derivative of the logarithmic attractant concentration.
The existence of the limiting gradient
G
C
and its scaling with
k
R
are explained by the underlying intracellular adaptation dynamics and the flagellar
motor response characteristics. For individual cells, we find that the overall average
run length in an exponential gradient is longer than that in a homogeneous environment,
which is caused by the constant kinase activity shift (decrease). The forward runs
(up the gradient) are longer than the backward runs, as expected; and depending on
the exact gradient, the (shorter) backward runs can be comparable to runs in a spatially
homogeneous environment, consistent with previous experiments. In (spatial) ligand
gradients that also vary in time, the chemotaxis motion is damped as the frequency
ω of the time-varying spatial gradient becomes faster than a critical value
ω
c
, which is controlled by the cell's chemotaxis adaptation rate
k
R
. Finally, our model, with no adjustable parameters, agrees quantitatively with the
classical capillary assay experiments where the attractant concentration changes both
in space and time. Our model can thus be used to study
E. coli chemotaxis behavior in arbitrary spatiotemporally varying environments. Further experiments
are suggested to test some of the model predictions.
. The linear dependence of
v
d
on
G =
d(ln[
L])/
dx directly demonstrates
E. coli's ability in sensing the derivative of the logarithmic attractant concentration.
The existence of the limiting gradient
G
C
and its scaling with
k
R
are explained by the underlying intracellular adaptation dynamics and the flagellar
motor response characteristics. For individual cells, we find that the overall average
run length in an exponential gradient is longer than that in a homogeneous environment,
which is caused by the constant kinase activity shift (decrease). The forward runs
(up the gradient) are longer than the backward runs, as expected; and depending on
the exact gradient, the (shorter) backward runs can be comparable to runs in a spatially
homogeneous environment, consistent with previous experiments. In (spatial) ligand
gradients that also vary in time, the chemotaxis motion is damped as the frequency
ω of the time-varying spatial gradient becomes faster than a critical value
ω
c
, which is controlled by the cell's chemotaxis adaptation rate
k
R
. Finally, our model, with no adjustable parameters, agrees quantitatively with the
classical capillary assay experiments where the attractant concentration changes both
in space and time. Our model can thus be used to study
E. coli chemotaxis behavior in arbitrary spatiotemporally varying environments. Further experiments
are suggested to test some of the model predictions.
Author Summary
A computational model, based on a coarse-grained description of the cell's underlying chemotaxis signaling pathway dynamics, is used to study Escherichia coli chemotactic motion in realistic environments that change in both space and time. We find that in an exponential attractant gradient, E. coli cells swim (randomly) toward higher attractant concentrations with a constant chemotactic drift velocity (CDV) that is proportional to the exponential gradient. In contrast, CDV continuously decreases in a linear gradient. These findings demonstrate that E. coli senses and responds to the relative gradient of the ligand concentration, instead of the gradient itself. The intracellular sensory adaptation rate does not affect the chemotactic motion directly; however, it sets a maximum relative ligand gradient beyond which CDV saturates. In time-varying environments, the E. coli's chemotactic motion is damped when the spatial gradient varies (in time) faster than a critical frequency determined by the adaptation rate. The run-length statistics of individual cells are studied and found to be consistent with previous experimental measurements. Finally, simulations of our model, with no adjustable parameters, agree quantitatively with the classical capillary assay in which the attractant concentration changes both in space and time. Our model can thus be used to predict and study E. coli chemotaxis behavior in arbitrary spatiotemporally varying environment.
Related collections
Most cited references45
- Record: found
- Abstract: not found
- Article: not found
Chemotaxis in Escherichia coli analysed by three-dimensional tracking.
- Record: found
- Abstract: found
- Article: not found