- Record: found
- Abstract: found
- Article: found
Combination treatment with the cap-dependent endonuclease inhibitor baloxavir marboxil and a neuraminidase inhibitor in a mouse model of influenza A virus infection
Read this article at
Abstract
Objectives
Baloxavir marboxil (formerly S-033188) is a first-in-class, orally available, cap-dependent endonuclease inhibitor licensed in Japan and the USA for the treatment of influenza virus infection. We evaluated the efficacy of delayed oral treatment with baloxavir marboxil in combination with a neuraminidase inhibitor in a mouse model of lethal influenza virus infection.
Methods
The inhibitory potency of baloxavir acid (the active form of baloxavir marboxil) in combination with neuraminidase inhibitors was tested in vitro. The therapeutic effects of baloxavir marboxil and oseltamivir phosphate, or combinations thereof, were evaluated in mice lethally infected with influenza virus A/PR/8/34; treatments started 96 h post-infection.
Results
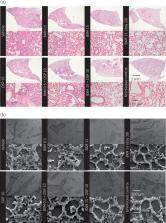
Combinations of baloxavir acid and neuraminidase inhibitor exhibited synergistic potency against viral replication by means of inhibition of cytopathic effects in vitro. In mice, baloxavir marboxil monotherapy (15 or 50 mg/kg twice daily) significantly and dose-dependently reduced virus titre 24 h after administration and completely prevented mortality, whereas oseltamivir phosphate treatments were not as effective. In this model, a suboptimal dose of baloxavir marboxil (0.5 mg/kg twice daily) in combination with oseltamivir phosphate provided additional efficacy compared with monotherapy in terms of virus-induced mortality, elevation of cytokine/chemokine levels and pathological changes in the lung.
Conclusions
Baloxavir marboxil monotherapy with 96 h-delayed oral dosing achieved drastic reductions in virus titre, inflammatory response and mortality in a mouse model. Combination treatment with baloxavir acid and oseltamivir acid in vitro and baloxavir marboxil and oseltamivir phosphate in mice produced synergistic responses against influenza virus infections, suggesting that treating humans with the combination may be beneficial.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors.
- Record: found
- Abstract: not found
- Article: not found
Neuraminidase inhibitors for influenza.
- Record: found
- Abstract: found
- Article: not found