- Record: found
- Abstract: found
- Article: found
Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier

Read this article at
Abstract
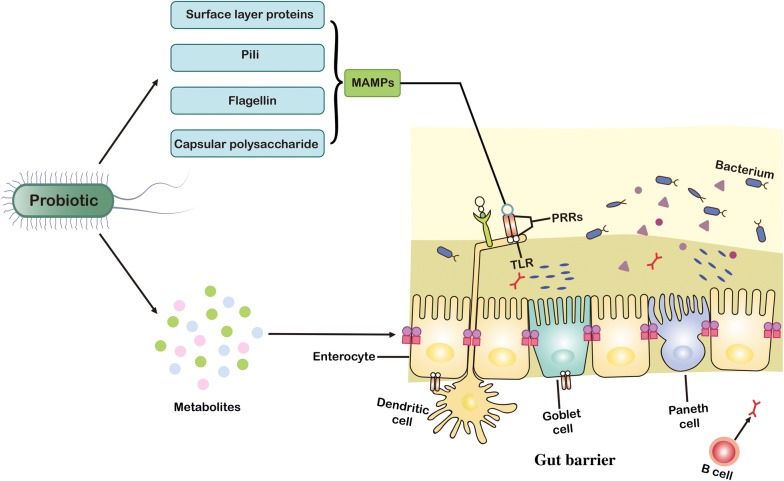
The gut microbiota can significantly affect the function of the intestinal barrier. Some intestinal probiotics (such as Lactobacillus, Bifidobacteria, a few Escherichia coli strains, and a new generation of probiotics including Bacteroides thetaiotaomicron and Akkermansia muciniphila) can maintain intestinal epithelial homeostasis and promote health. This review first summarizes probiotics’ regulation of the intestinal epithelium via their surface compounds. Surface layer proteins, flagella, pili and capsular polysaccharides constitute microbial-associated molecular patterns and specifically bind to pattern recognition receptors, which can regulate signaling pathways to produce cytokines or inhibit apoptosis, thereby attenuating inflammation and enhancing the function of the gut epithelium. The review also explains the effects of metabolites (such as secreted proteins, organic acids, indole, extracellular vesicles and bacteriocins) of probiotics on host receptors and the mechanisms by which these metabolites regulate gut epithelial barrier function. Previous reviews summarized the role of the surface macromolecules or metabolites of gut microbes (including both probiotics and pathogens) in human health. However, these reviews were mostly focused on the interactions between these substances and the intestinal mucosal immune system. In the current review, we only focused on probiotics and discussed the molecular interaction between these bacteria and the gut epithelial barrier.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon.
- Record: found
- Abstract: found
- Article: not found
Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4.
- Record: found
- Abstract: found
- Article: not found