- Record: found
- Abstract: found
- Article: found
Temperature-Controlled Syngas Production via Electrochemical CO 2 Reduction on a CoTPP/MWCNT Composite in a Flow Cell

Read this article at
Abstract
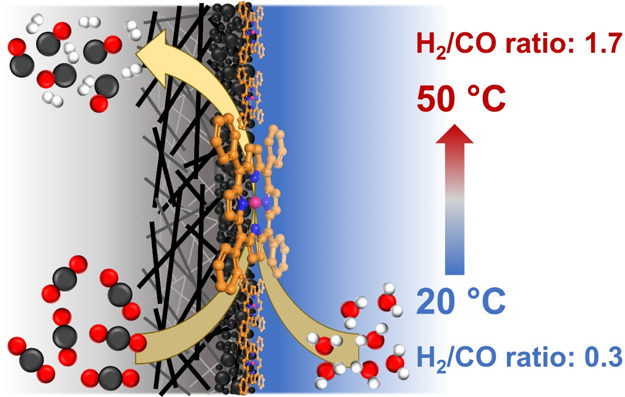
The mixture of CO and H 2, known as syngas, is a building block for many substantial chemicals and fuels. Electrochemical reduction of CO 2 and H 2O to syngas would be a promising alternative approach for its synthesis due to negative carbon emission footprint when using renewable energy to power the reaction. Herein, we present temperature-controlled syngas production by electrochemical CO 2 and H 2O reduction on a cobalt tetraphenylporphyrin/multiwalled carbon nanotube (CoTPP/MWCNT) composite in a flow cell in the temperature range of 20–50 °C. The experimental results show that for all the applied potentials the ratio of H 2/CO increases with increasing temperature. Interestingly, at −0.6 V RHE and 40 °C, the H 2/CO ratio reaches a value of 1.2 which is essential for the synthesis of oxo-alcohols. In addition, at −1.0 V RHE and 20 °C, the composite shows very high selectivity toward CO formation, reaching a Faradaic efficiency of ca. 98%. This high selectivity of CO formation is investigated by density functional theory modeling which underlines that the potential-induced oxidation states of the CoTPP catalyst play a vital role in the high selectivity of CO production. Furthermore, the stability of the formed intermediate species is evaluated in terms of the pK a value for further reactions. These experimental and theoretical findings would provide an alternative way for syngas production and help us to understand the mechanism of molecular catalysts in dynamic conditions.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy.
- Record: found
- Abstract: not found
- Article: not found
The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals
- Record: found
- Abstract: found
- Article: not found