- Record: found
- Abstract: found
- Article: found
Obesity-induced inflammation: The impact of the hematopoietic stem cell niche
Read this article at
Abstract
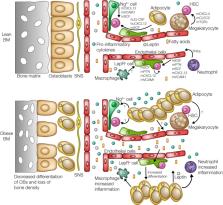
Obesity and obesity-related diseases like type 2 diabetes (T2D) are prominent global health issues; therefore, there is a need to better understand the mechanisms underlying these conditions. The onset of obesity is characterized by accumulation of proinflammatory cells, including Ly6c hi monocytes (which differentiate into proinflammatory macrophages) and neutrophils, in metabolic tissues. This shift toward chronic, low-grade inflammation is an obese-state hallmark and highly linked to metabolic disorders and other obesity comorbidities. The mechanisms that induce and maintain increased inflammatory myelopoiesis are of great interest, with a recent focus on how obesity affects more primitive hematopoietic cells. The hematopoietic system is constantly replenished by proper regulation of hematopoietic stem and progenitor (HSPC) pools in the BM. While early research suggests that chronic obesity promotes expansion of myeloid-skewed HSPCs, the involvement of the hematopoietic stem cell (HSC) niche in regulating obesity-induced myelopoiesis remains undefined. In this review, we explore the role of the multicellular HSC niche in hematopoiesis and inflammation, and the potential contribution of this niche to the hematopoietic response to obesity. This review further aims to summarize the potential HSC niche involvement as a target of obesity-induced inflammation and a driver of obesity-induced myelopoiesis.
Related collections
Most cited references199
- Record: found
- Abstract: found
- Article: not found
MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity.
- Record: found
- Abstract: found
- Article: not found
Obesity induces a phenotypic switch in adipose tissue macrophage polarization.
- Record: found
- Abstract: found
- Article: not found