- Record: found
- Abstract: found
- Article: found
Isoniazid metabolism and hepatotoxicity

Read this article at
Abstract
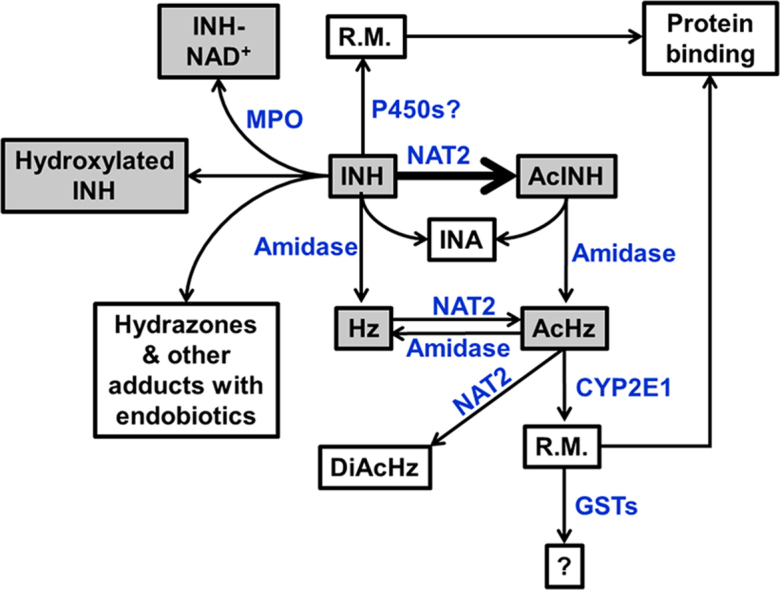
Isoniazid (INH) is highly effective for the management of tuberculosis. However, it can cause liver injury and even liver failure. INH metabolism has been thought to be associated with INH-induced liver injury. This review summarized the metabolic pathways of INH and discussed their associations with INH-induced liver injury.
Graphical abstract
Isoniazid (INH) is highly effective for the management of tuberculosis. However, it can cause liver injury and even liver failure. INH metabolism has been thought to be associated with INH-induced liver injury. This review summarized the metabolic pathways of INH and discussed their associations with INH-induced liver injury.
Related collections
Most cited references130
- Record: found
- Abstract: found
- Article: not found
Role of CYP2E1 in the hepatotoxicity of acetaminophen.
- Record: found
- Abstract: found
- Article: not found
Oxidative stress, toxicology, and pharmacology of CYP2E1.
- Record: found
- Abstract: found
- Article: not found