- Record: found
- Abstract: found
- Article: found
Six-Month Persistence and Multi-domain Effectiveness of Guselkumab in Adults with Psoriatic Arthritis: Real-World Data from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry

Read this article at
Abstract
Introduction
The aim of this work is to evaluate treatment persistence and clinical outcomes after 6 months of on-label guselkumab use in patients with rheumatologist-diagnosed active psoriatic arthritis (PsA) enrolled in the CorEvitas PsA/Spondyloarthritis Registry.
Methods
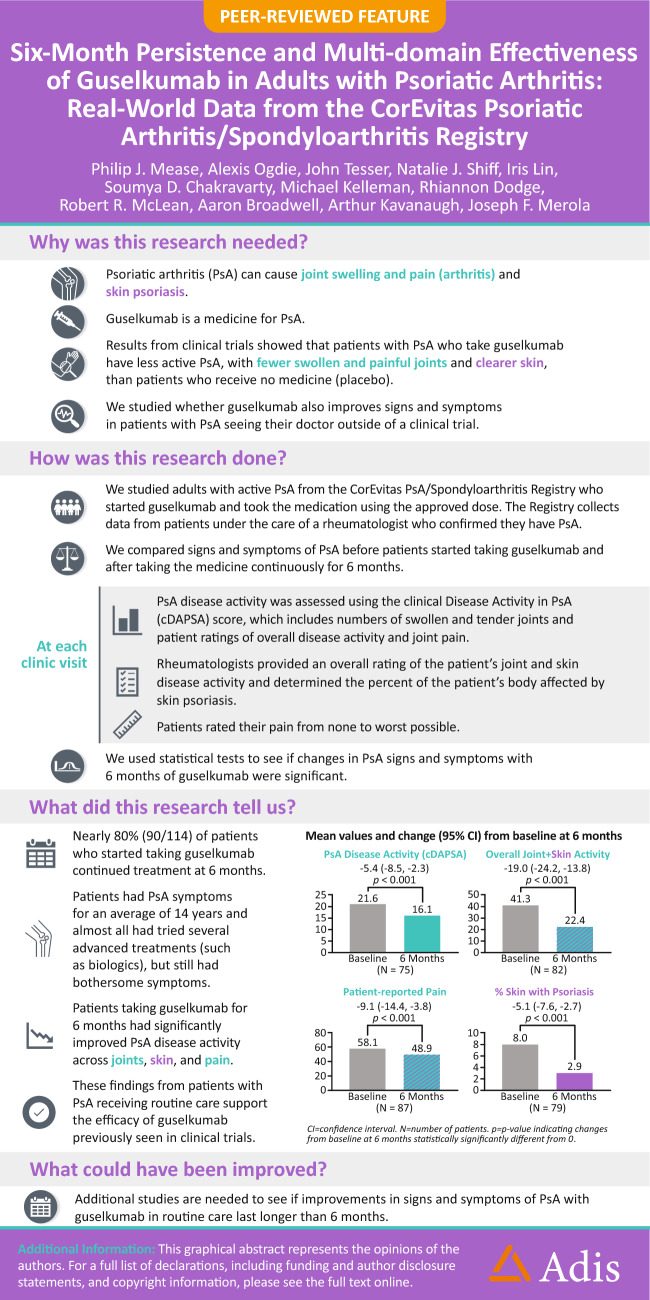
Participants with PsA who initiated and persisted with on-label guselkumab use post-Food and Drug Administration (FDA) approval for active PsA (7/13/2020; subcutaneous 100 mg at weeks 0, 4, and every 8 weeks) at their 6-month follow-up visit (occurring through 3/31/2023) comprised the primary analysis population (On-Label Persisters). Hierarchical, multiplicity-controlled primary and secondary outcomes were mean (95% confidence interval) changes from baseline at 6 months in clinical Disease Activity Index for PsA (cDAPSA; primary), Physician Global Assessment (PGA) of arthritis and psoriasis (visual analog scale [VAS] 0–100), patient-reported pain (VAS 0–100), and percent body surface area with psoriasis (%BSA). Paired t tests determined changes that were statistically significantly different from 0 ( α = 0.05).
Results
Among 114 patients who initiated on-label guselkumab and had eligible baseline and 6-month visits, 90 (78.9%) had persistent use. Among these On-Label Persisters at baseline, mean duration of PsA symptoms = 13.6 years; mean cDAPSA, PGA, and patient-reported pain = 22.0, 42.3, and 57.0, respectively; 94.4% had a history of psoriasis (mean BSA 7.6%); and 18.9% and 73.3%, respectively, previously received 1 or ≥ 2 biologic/targeted synthetic disease-modifying antirheumatic drugs. The mean change (improvement) in cDAPSA was − 5.4 (− 8.5, − 2.3; p < 0.001) at 6 months. Significant mean improvements in PGA (− 19.0 [− 24.2, − 13.8]), patient-reported pain (− 9.1 [− 14.4, − 3.8]), and %BSA (− 5.1 [− 7.6, − 2.7]) were also observed (all p < 0.001).
Conclusions
In this real-world PsA population, generally characterized by longstanding, treatment-resistant, active disease at baseline, persistent guselkumab use in nearly 80% of patients with on-label use was accompanied by significant improvements in joint and skin symptoms and patient-reported pain at 6 months. These registry data support results from randomized clinical trials demonstrating the efficacy of guselkumab in improving PsA signs and symptoms.
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update
- Record: found
- Abstract: found
- Article: not found
Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis.
- Record: found
- Abstract: found
- Article: not found