- Record: found
- Abstract: found
- Article: found
PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers
Read this article at
Summary
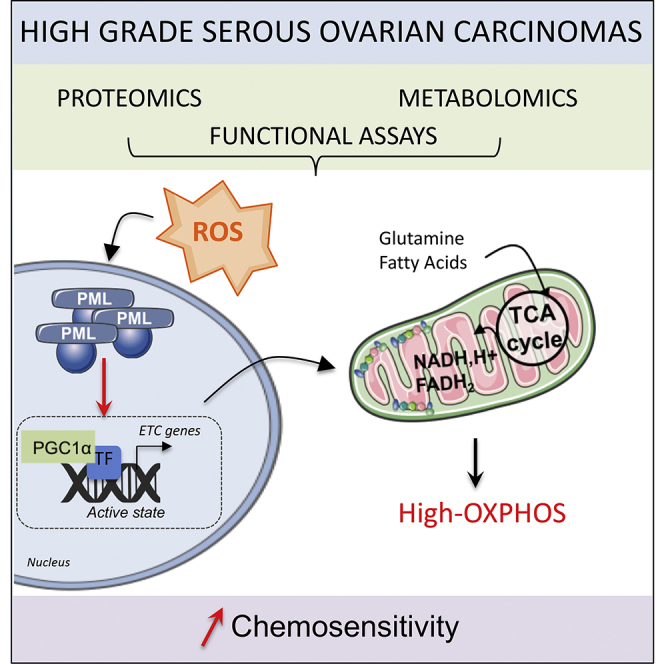
High-grade serous ovarian cancer (HGSOC) remains an unmet medical challenge. Here, we unravel an unanticipated metabolic heterogeneity in HGSOC. By combining proteomic, metabolomic, and bioergenetic analyses, we identify two molecular subgroups, low- and high-OXPHOS. While low-OXPHOS exhibit a glycolytic metabolism, high-OXPHOS HGSOCs rely on oxidative phosphorylation, supported by glutamine and fatty acid oxidation, and show chronic oxidative stress. We identify an important role for the PML-PGC-1α axis in the metabolic features of high-OXPHOS HGSOC. In high-OXPHOS tumors, chronic oxidative stress promotes aggregation of PML-nuclear bodies, resulting in activation of the transcriptional co-activator PGC-1α. Active PGC-1α increases synthesis of electron transport chain complexes, thereby promoting mitochondrial respiration. Importantly, high-OXPHOS HGSOCs exhibit increased response to conventional chemotherapies, in which increased oxidative stress, PML, and potentially ferroptosis play key functions. Collectively, our data establish a stress-mediated PML-PGC-1α-dependent mechanism that promotes OXPHOS metabolism and chemosensitivity in ovarian cancer.
Graphical Abstract
Highlights
-
•
High-grade serous ovarian cancers display OXPHOS metabolic heterogeneity
-
•
High-OXPHOS show high electron transport chain synthesis and respiration rate
-
•
Oxidative stress in high-OXPHOS HGSOC activates PML-PGC-1α and ETC transcription
-
•
High-OXPHOS HGSOCs show enhanced chemosensitivity through oxidative stress and PML
Abstract
Gentric et al. identify metabolically heterogeneous OXPHOS subgroups in high-grade serous ovarian cancers (HGSOCs). In high-OXPHOS tumors, chronic oxidative stress promotes aggregation of PML-nuclear bodies that activate PGC-1α, electron transport chain synthesis, and mitochondrial respiration. High-OXPHOS HGSOCs exhibit chemosensitivity, in which increased oxidative stress and PML play key functions.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: found
MYC/PGC-1α Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells.

- Record: found
- Abstract: found
- Article: found
Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer
- Record: found
- Abstract: found
- Article: not found