- Record: found
- Abstract: found
- Article: not found
Development of a gut microbe-targeted non-lethal therapeutic to inhibit thrombosis potential
Read this article at
Abstract
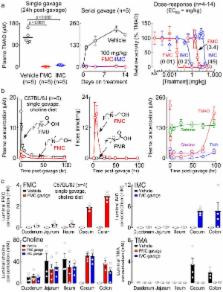
Trimethylamine-N-oxide (TMAO), a microbiota-dependent metabolite derived from trimethylamine (TMA)-containing nutrients that are abundant in a Western diet, enhances both platelet responsiveness and in vivo thrombosis potential in animal models and predicts incident atherothrombotic event risks in clinical studies. Here, utilizing a mechanism-based inhibitor approach targeting a major microbial TMA-generating enzyme (CutC/D), we developed potent, time-dependent and irreversible inhibitors that do not affect commensal viability. In animal models, a single oral dose of a CutC/D inhibitor significantly reduced plasma TMAO levels for up to 3 days and rescued diet-induced enhanced platelet responsiveness and thrombus formation, without observable toxicity or increased bleeding risk. The inhibitor selectively accumulated within intestinal microbes to millimolar levels, a concentration over a million-fold higher than needed for a therapeutic effect. These studies reveal that mechanism-based inhibition of gut microbial TMA/TMAO production reduces thrombosis potential, a critical adverse complication in heart disease. They also offer a generalizable approach for the selective non-lethal targeting of gut microbial enzymes linked to host disease, while limiting systemic exposure of the inhibitor in the host.
Related collections
Most cited references34

- Record: found
- Abstract: found
- Article: found
Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide
- Record: found
- Abstract: found
- Article: not found
Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide.
- Record: found
- Abstract: found
- Article: not found
