- Record: found
- Abstract: found
- Article: found
Development and testing of a random forest-based machine learning model for predicting events among breast cancer patients with a poor response to neoadjuvant chemotherapy
Read this article at
Abstract
Background
Breast cancer (BC) is the most common malignant tumor around the world. Timely detection of the tumor progression after treatment could improve the survival outcome of patients. This study aimed to develop machine learning models to predict events (defined as either (1) the first tumor relapse locally, regionally, or distantly; (2) a diagnosis of secondary malignant tumor; or (3) death because of any reason.) in BC patients post-treatment.
Methods
The patients with the response of stable disease (SD) and progressive disease (PD) after neoadjuvant chemotherapy (NAC) were selected. The clinicopathological features and the survival data were recorded in 1 year and 5 years, respectively. Patients were randomly divided into the training set and test set in the ratio of 8:2. A random forest (RF) and a logistic regression were established in both of 1-year cohort and the 5-year cohort. The performance was compared between the two models. The models were validated using data from the Surveillance, Epidemiology, and End Results (SEER) database.
Results
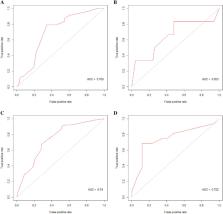
A total of 315 patients were included. In the 1-year cohort, 197 patients were divided into a training set while 87 were into a test set. The specificity, sensitivity, and AUC were 0.800, 0.833, and 0.810 in the RF model. And 0.520, 0.833, and 0.653 of the logistic regression. In the 5-year cohort, 132 patients were divided into the training set while 33 were into the test set. The specificity, sensitivity, and AUC were 0.882, 0.750, and 0.829 in the RF model. And 0.882, 0.688, and 0.752 of the logistic regression. In the external validation set, of the RF model, the specificity, sensitivity, and AUC were 0.765, 0.812, and 0.779. Of the logistics regression model, the specificity, sensitivity, and AUC were 0.833, 0.376, and 0.619.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1).
- Record: found
- Abstract: found
- Article: not found